Accelerator capable of improving functionalization process of adoptive DCs (Dendritic Cells), as well as preparation method and application of accelerator
A dendritic cell and enhancer technology, which is applied in the field of enhancer and its preparation for improving the functionalization process of adoptive dendritic cells, can solve the problem of low response rate, inability to effectively promote adoptive DCs to secrete IL-12, etc. problems, to achieve the effect of good biological safety and increase the possibility of clinical promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1, the preparation of spherical gold nanoparticles
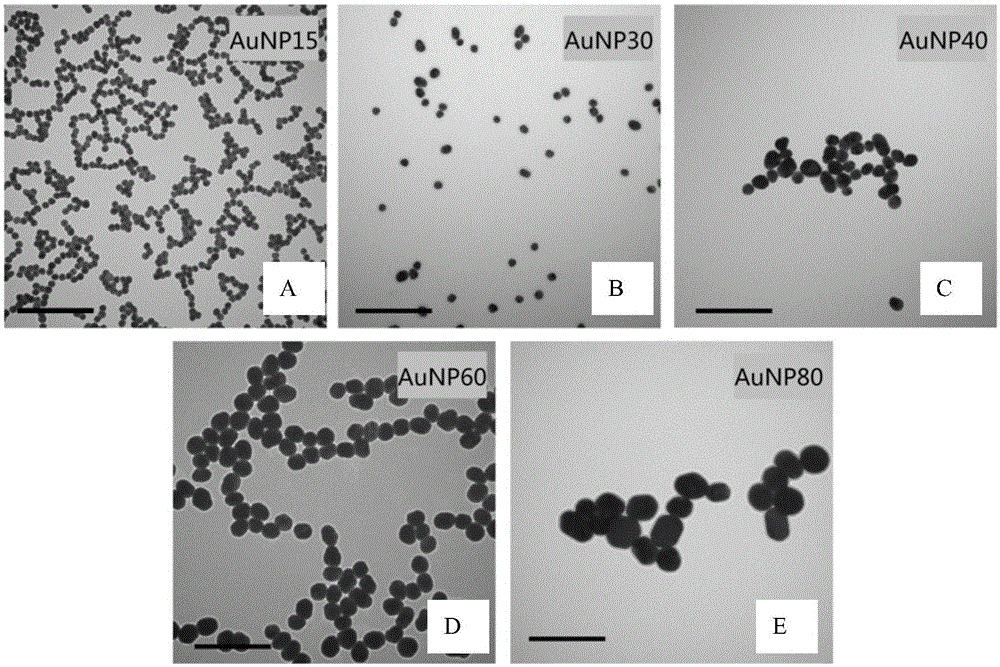
[0064] Place a three-necked flask containing 100mL of 0.01wt% chloroauric acid solution in an oil bath with stirring and heating to 130°C, quickly add 0.4-3.0mL of 1wt% sodium citrate solution, and keep stirring at this temperature for half an hour, Obtain the colloidal solution, centrifuge and concentrate at 3000-12000rpm for 10-20min, discard the supernatant, and obtain spherical gold nanoparticles, such as figure 1 shown. from figure 1 It can be seen from the transmission electron microscope photos of spherical gold nanoparticles that the hydrodynamic radius of the spherical gold nanoparticles is in the range of 10nm-90nm (gold nanoparticles are abbreviated as "AuNP+radius", and A-E are the hydrodynamic radii of 15nm, 30nm, 40nm, 60nm and 80nm spherical gold nanoparticles), and the distribution is relatively uniform (the scale in the figure is 200nm). In industrial applications, the scale of preparati...
Embodiment 2
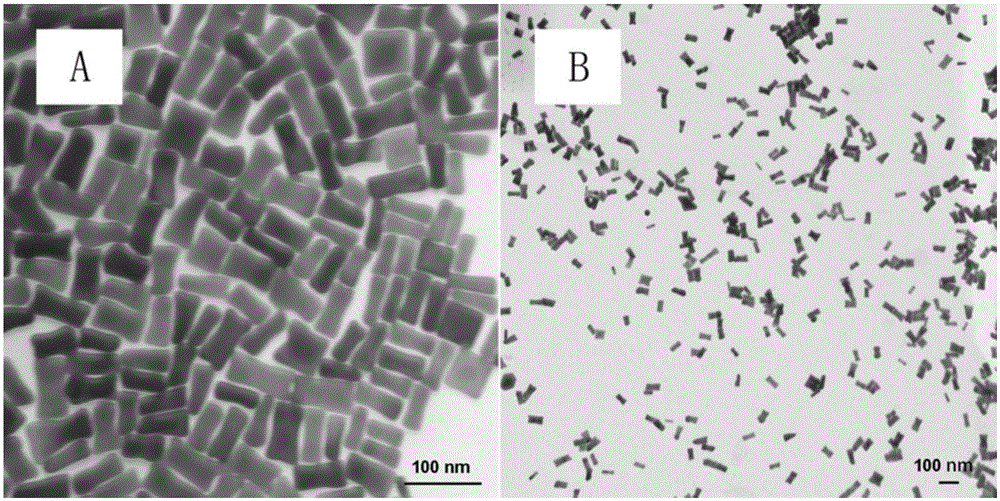
[0065] Embodiment 2, preparation of rod-shaped gold nanoparticles
[0066] Ⅰ. Add 0.25mL0.01mol / L chloroauric acid solution to 7.5mL0.1mol / L cetyltrimethylammonium bromide (CTAB) solution, mix well and then add 0.6mL0.01mol / L The freshly prepared sodium borohydride solution placed in an ice-water bath was inverted back and forth for 2 minutes to mix well to obtain a bright brown-yellow gold nanometer seed solution. The gold nanometer seed solution was aged in a water bath at 25° C. for 3 h.
[0067]Ⅱ. Add 4mL of 10mmol / L chloroauric acid solution, 0.6mL of 10mmol / L silver nitrate solution and 0.64mL of 0.01mol / L ascorbic acid solution to 95mL of 0.01mol / L CTAB solution in turn, and then add 0.1mL step Ⅰ Mix the aged gold nano seed solution, put it in a water bath at 25°C for 3 hours, and obtain a colloidal solution of rod-shaped gold nanoparticles (stock solution); centrifuge the stock solution at least twice at 8000 rpm, each time 10min, to remove excess CTAB, centrifuge an...
Embodiment 3
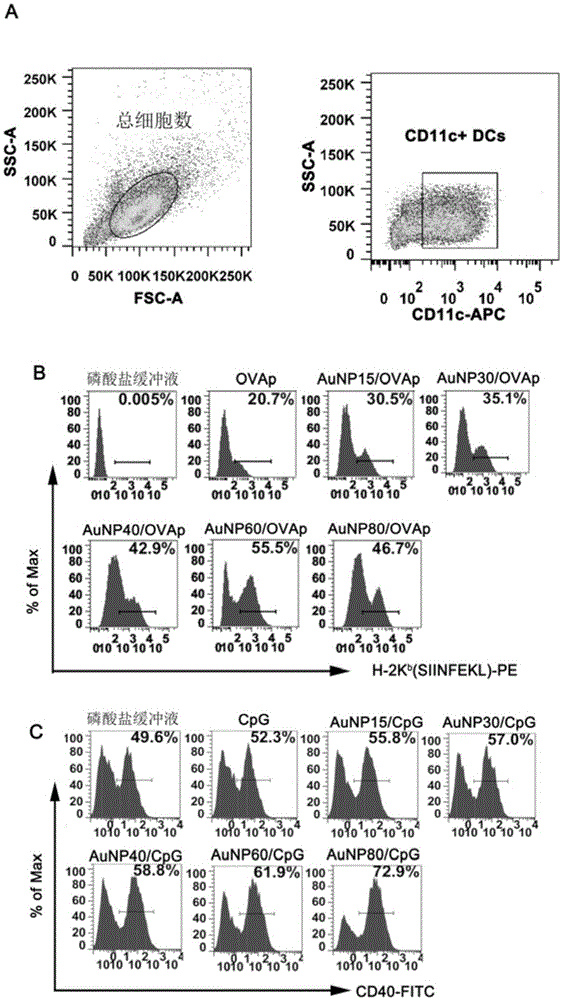
[0068] Embodiment three, the preparation of CpG-OVAp spherical nano-gold cocktail
[0069] 1. Functional modification of spherical gold nanoparticles by Toll-like receptor 9 (TLR9) pathway stimulator oligonucleotide single strand (CpG):
[0070] (1) The reducing agent dithiothreitol (DTT) performs dedisulfide bond pretreatment on the thiolated CpG sequence (5'-SH-AAAAA-Spacer9-tccatgacgttcctgacgtt-3'):
[0071] Prepare 160 μL of DTT solution with a DTT concentration of 0.125 mol / L in phosphate buffered saline (PBS) with pH=8.0, 0.18 mol / L, add 40 μL of 100 μmol / L CpG into the DTT solution, react at room temperature for 1 hour, and pass through a nucleic acid purification column (GE Healthcare Life Science, NAP TM -5columes) for desalination and other impurity removal treatments.
[0072] (2), CpG functional modification of spherical gold nanoparticles:
[0073] Add the reaction solution after the impurity removal treatment in step (1) to the spherical gold nanoparticles obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com