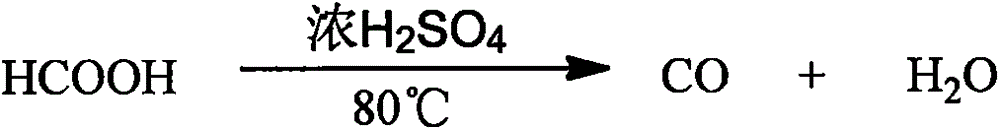Method for industrially preparing high-purity carbon monoxide through formic acid dehydration
A carbon monoxide and formic acid technology, applied in directions such as carbon monoxide, can solve the problems of low carbon monoxide purity, inability to continuously produce, and high production costs, and achieve the effects of safe, stable and reliable operation, saving purchase costs, and reducing purchase costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
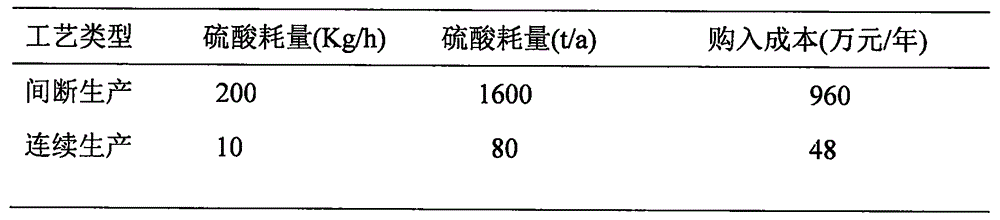
[0052] (1) Dehydration reaction: 84% formic acid is divided into three paths with a speed of 256Kg / h by a metering pump and dripped into the 80% concentrated sulfuric acid in the dehydration tower tray of the dehydration reactor at the same time. After cooling, the concentrated sulfuric acid enters the dehydration tower again at 500Kg / h to dehydrate with formic acid at 75°C to generate CO and H 2 O; Concentrated sulfuric acid must supplement evaporation loss of 5%, namely: 10Kg / h.
[0053] (2) The carbon monoxide gas generated in step (1) is defoamed by the wire mesh demister made of PTFE material at the top of the dehydration tower, and defoamed by the wire mesh demister made of S30408 material in the alkali washing tower, and then enters the alkali washing containing NaOH aqueous solution The tower neutralizes the saturated acidic water vapor in the carbon monoxide gas, and the neutral carbon monoxide is then passed through a chiller at a temperature of 2-3°C to remove fre...
Embodiment 2
[0058] Except for the following parameters, the method and process used are the same as in Example 1.
[0059] Concentration of formic acid: 87%
[0060] Dehydration reaction temperature: 78°C
[0061] The rate at which the concentrated sulfuric acid enters the dehydration reaction tower is: 550Kg / h
[0062] The temperature to which the concentrated sulfuric acid is cooled is: 75°C
[0063] Finally, after purification, carbon monoxide with a purity of 99.999% was obtained, and the conversion rate of formic acid was 62.8%.
Embodiment 3
[0065] Except for the following parameters, the method and process used are the same as in Example 1.
[0066] Concentration of formic acid: 90%
[0067] Dehydration reaction temperature: 80°C
[0068] The rate at which the concentrated sulfuric acid enters the dehydration reaction tower is: 600Kg / h
[0069] The temperature to which the concentrated sulfuric acid is cooled is: 80°C
[0070] Finally, after purification, carbon monoxide with a purity of 99.99% was obtained, and the conversion rate of formic acid was 61.8%.
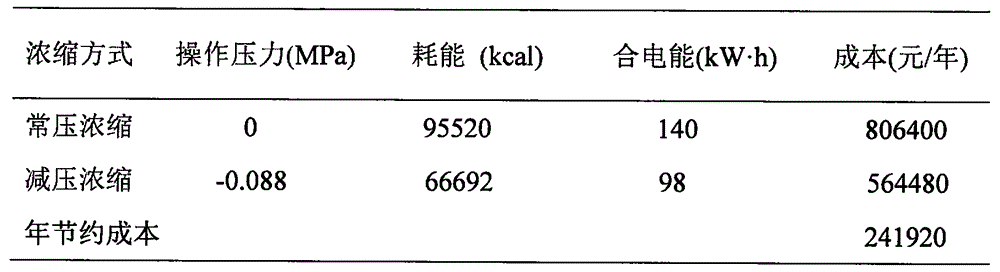
[0071] According to the acidity degree and temperature conditions of each stage, the present invention selects materials and customizes equipment to meet the requirements of working conditions, and achieves safe and reliable production, stable and simple operation, environmental protection, energy saving, low cost, high purity of carbon monoxide obtained, and high conversion rate of formic acid . The invention can generate remarkable benefits and has goo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com