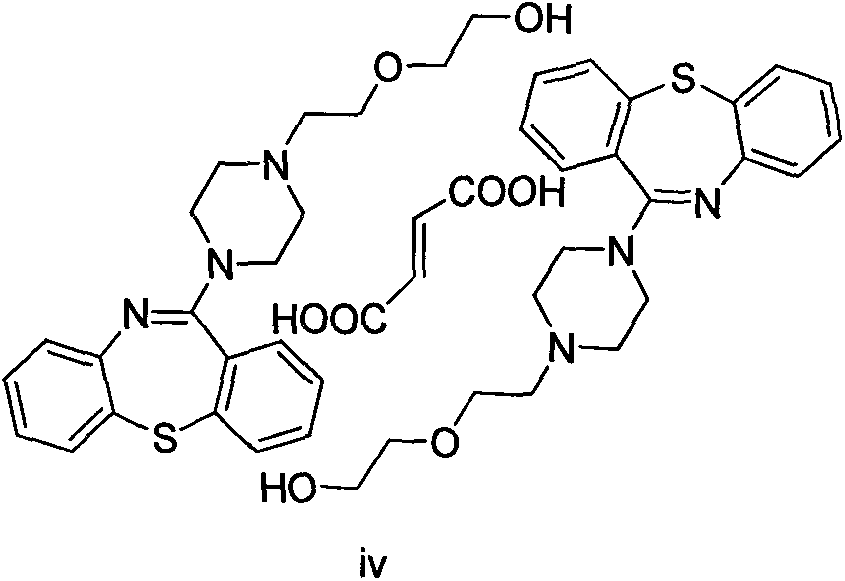
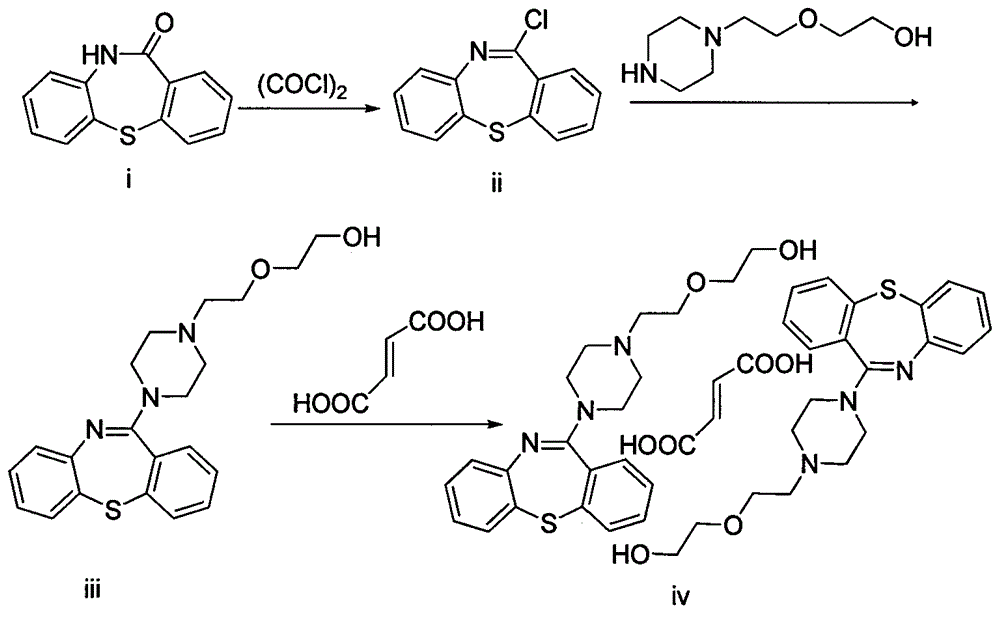
Preparation method for crystalline dibenzothiazepine derivative
A technology of benzothiazepine and thiazepine, which is applied in the field of chemical drug synthesis, can solve the problems of harsh production conditions, high price and high production cost, and achieve the effects of reducing pollution, reducing the generation of by-products and improving the reaction rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]Add toluene 200g in the 500ml reaction flask, add dibenzo[b,f][1,4]thiazepin-11-[10H]ketone (50.0g, 0.22mol), triethylamine (33.4g , 0.33mol), N,N-dimethylformamide 12g and oxalyl chloride (41.9g, 0.33mol), the temperature was raised to 100-105°C, and the reaction was kept for 6 hours until the reaction was complete. After the reaction is complete, lower the temperature to 60°C, keep warm at 60-65°C and distill under reduced pressure for 1 hour, then cool the frozen brine to 0°C, add the cooled reaction solution to 330 g of ice water, separate the layers, collect the organic layer and dry it with anhydrous magnesium sulfate to obtain 250 g of 11-chloro-dibenzo[b,f][1,4]thiazepine toluene solution.
[0028] Add 250 g of the obtained 11-chloro-dibenzo[b,f][1,4]thiazepine toluene solution into a 500ml reaction flask, and add potassium carbonate (60.0g, 0.40mol), benzyltriethyl Ammonium chloride (2.3 g, 0.01 mol) was heated to 100° C., and the reaction was kept for 0.5 hour...
Embodiment 2
[0032] Add 300 g of toluene to a 500 ml reaction flask, and add dibenzo[b, f][1,4]thiazepin-11-[10H]one (50.0 g, 0.22 mol), N, N-diiso Propylethylamine (28.4g, 0.22mol), 8g of N,N-dimethylformamide and oxalyl chloride (55.8g, 0.44mol) were heated up to 100-105°C and kept for 4 hours until the reaction was complete. After the reaction is complete, lower the temperature to 60°C, keep warm at 60-65°C and distill under reduced pressure for 1 hour, then cool the frozen brine to 0°C, add the cooled reaction solution to 400g of ice water, separate the layers, collect the organic layer and dry it with anhydrous magnesium sulfate to obtain 350 g of 11-chloro-dibenzo[b,f][1,4]thiazepine toluene solution.
[0033] Add 350 g of the obtained 11-chloro-dibenzo[b,f][1,4]thiazepine toluene solution into a 100ml reaction flask, add anhydrous sodium carbonate (42.4g, 0.40mol) under stirring, tetrabutyl Ammonium bromide (6.4g, 0.02mol), the temperature was raised to 100°C, and the reaction was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com