Absorbable medical suture with good degradation resistance and compatibility and preparation method thereof
A compatible and degradation-resistant technology, applied in the field of medical sutures, it can solve the problems of slow degradation rate, supply, and easy cell adhesion, and achieve the effects of increasing smoothness, increasing compatibility, and improving cell affinity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
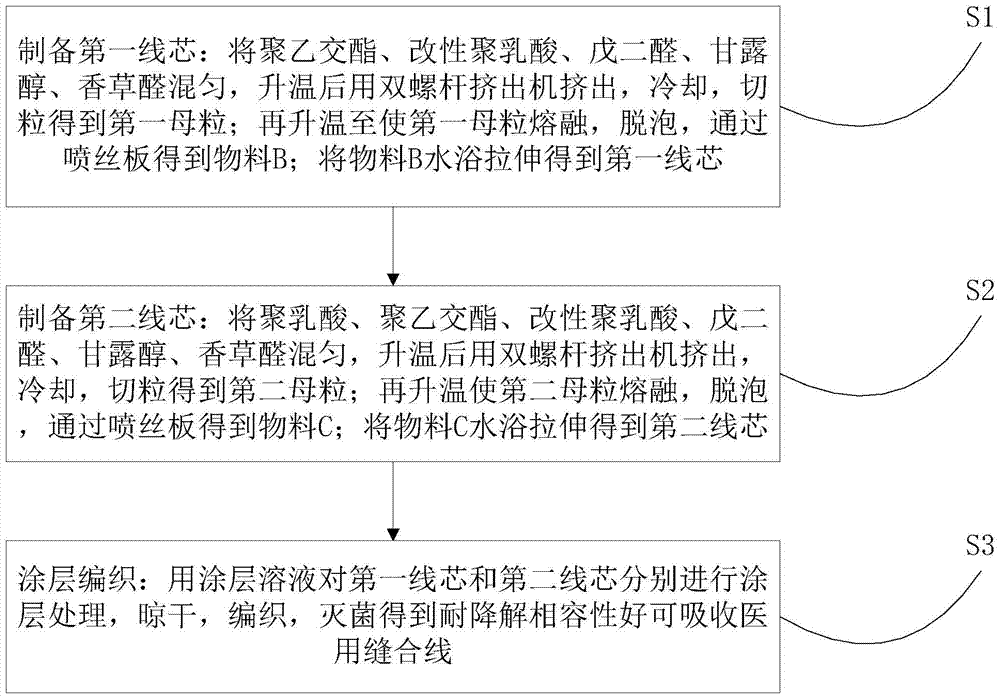
[0036] refer to figure 1 A method for preparing absorbable medical sutures with good degradation resistance and compatibility proposed by the present invention comprises the following steps:
[0037] S1. Preparation of the first wire core: mix polyglycolide, modified polylactic acid, glutaraldehyde, mannitol, and vanillin, extrude with a twin-screw extruder after heating up, cool, and pelletize to obtain the first mother core. Then heat up to melt the first masterbatch, defoam, and obtain material B through a spinneret; stretch material B in a water bath to obtain the first wire core;
[0038]S2. Preparation of the second wire core: mix polylactic acid, polyglycolide, modified polylactic acid, glutaraldehyde, mannitol, and vanillin, extrude with a twin-screw extruder after heating up, cool, and pelletize to obtain the second core. Second masterbatch; heating again to melt the second masterbatch, defoaming, and obtaining material C through a spinneret; stretching material C in...
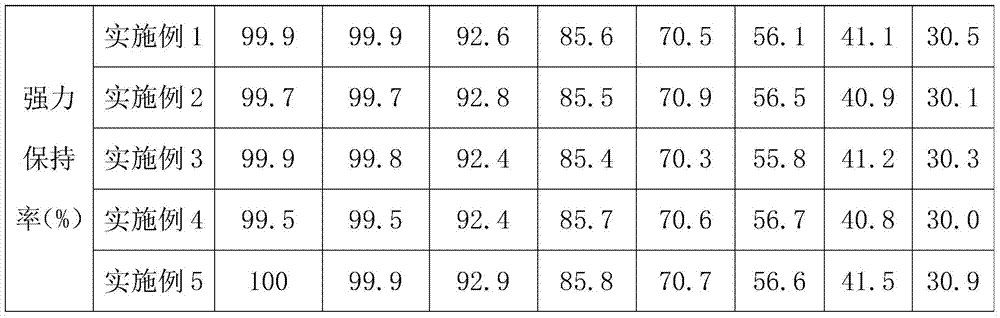
Embodiment 1
[0042] An absorbable medical suture with good degradation resistance and compatibility, braided from at least one first fiber and / or second fiber, wherein the first fiber includes a first core and a second core coated on the outside of the first core. A coating, the second fiber includes a second core and a second coating coated on the outside of the second core;
[0043] The raw materials of the first core include by weight: 50 parts of polyglycolide, 10 parts of modified polylactic acid, 3 parts of glutaraldehyde, 4 parts of mannitol, and 3 parts of vanillin;
[0044] The raw materials of the second core include by weight: 60 parts of polylactic acid, 10 parts of polyglycolide, 20 parts of modified polylactic acid, 4 parts of glutaraldehyde, 5 parts of mannitol, and 4 parts of vanillin;
[0045] Wherein, the preparation method of the modified polylactic acid is as follows: add 12 parts of itaconic acid to 35 parts of benzoyl chloride in parts by weight, heat up to 150 ° C, k...
Embodiment 2
[0052] An absorbable medical suture with good degradation resistance and compatibility, braided from at least one first fiber and / or second fiber, wherein the first fiber includes a first core and a second core coated on the outside of the first core. A coating, the second fiber includes a second core and a second coating coated on the outside of the second core;
[0053] The raw materials of the first core include by weight: 70 parts of polyglycolide, 5 parts of modified polylactic acid, 5 parts of glutaraldehyde, 2 parts of mannitol, and 5 parts of vanillin;
[0054] The raw materials of the second core include by weight: 40 parts of polylactic acid, 20 parts of polyglycolide, 10 parts of modified polylactic acid, 6 parts of glutaraldehyde, 3 parts of mannitol, and 6 parts of vanillin;
[0055] Wherein, the preparation method of the modified polylactic acid is as follows: add 8 parts of itaconic acid to 45 parts of benzoyl chloride in parts by weight, heat up to 140 ° C, kee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com