A kind of method of synthesizing diselenide
A technology of diselenide and selenium powder, applied in organic chemistry and other directions, can solve the problems of not conforming to the spirit of green chemistry, harsh reaction conditions, and difficulty in large-scale production, and achieve simple reaction, low equipment corrosion, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1: Add 252 mg (2 mmol) of anhydrous sodium sulfite and 158 mg (2 mmol) of selenium powder into the high-pressure sealed tube, vacuumize and fill with nitrogen, then add 2 ml of water into the high-pressure sealed tube, and then vacuumize Then fill it with nitrogen, then place it in an oil bath at 100°C and stir for 10 hours to react to obtain sodium selenosulfate.
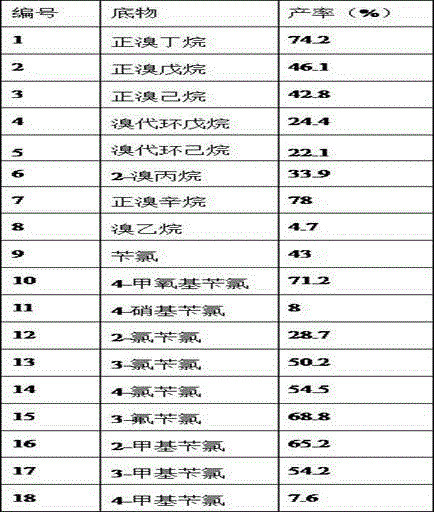
[0020] Step 2: Mix 137 mg (1 mmol) of n-bromobutane, 1 ml of ethanol and the whole amount of sodium selenosulfate prepared above in another high-pressure sealed tube, and stir in an oil bath at 140°C for 10 hours. After the reaction, it was extracted with ether and separated by climbing a board to obtain the product—diselenide, with a yield of 74.2%.
[0021] The above purification method can also adopt column chromatography or thin layer chromatography.
Embodiment 2
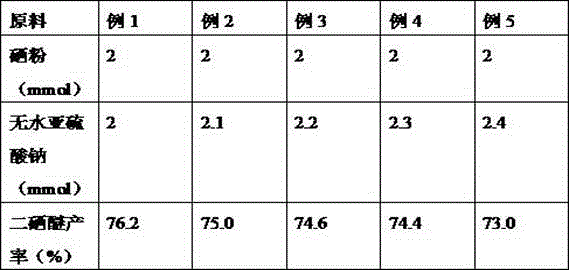
[0023] Other conditions are the same as embodiment 1, check the impact of different temperatures on the reaction in step 1), and the experimental results are shown in the table below:
[0024]
[0025] It can be seen from the above table that the yield is higher when the reaction temperature is 140° C. (Example 1).
Embodiment 3
[0027] Other conditions are the same as embodiment 1, check step 2) in different reaction temperatures, investigate the change result of final yield, as shown in the table below:
[0028]
[0029] It can be seen from the above table that the yield is the highest when the reaction temperature is 100°C (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com