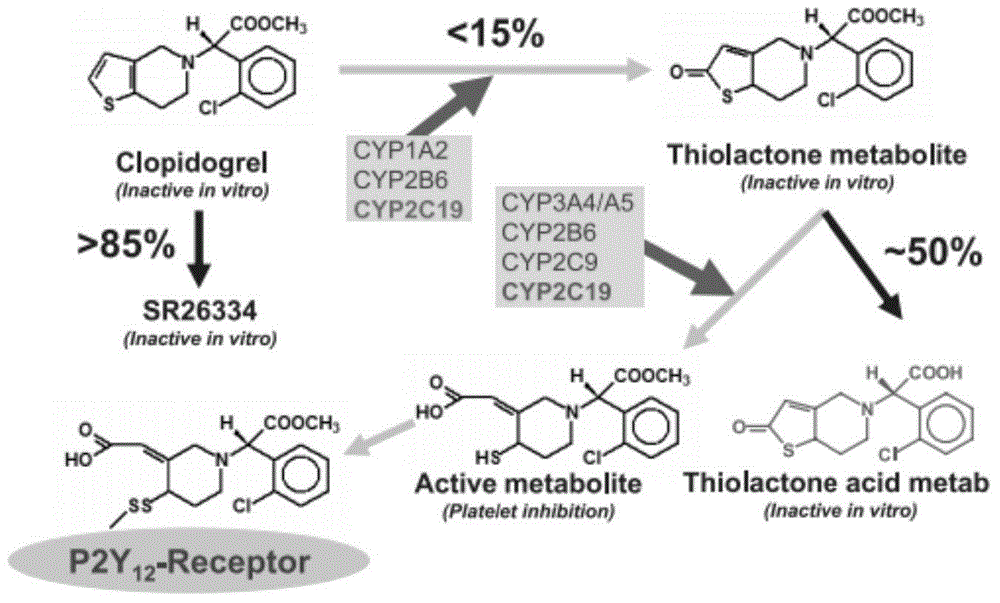
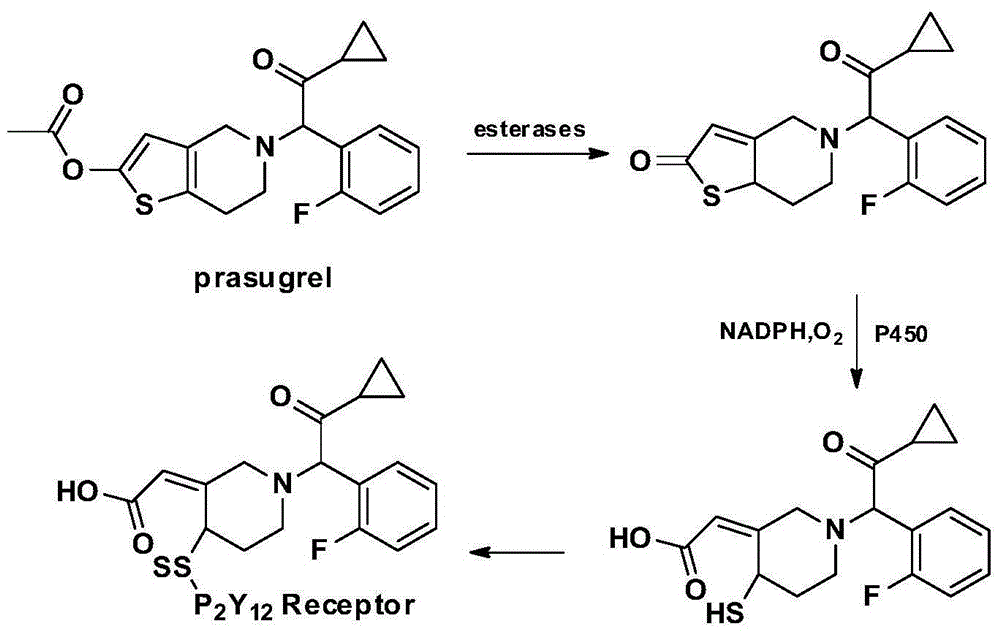
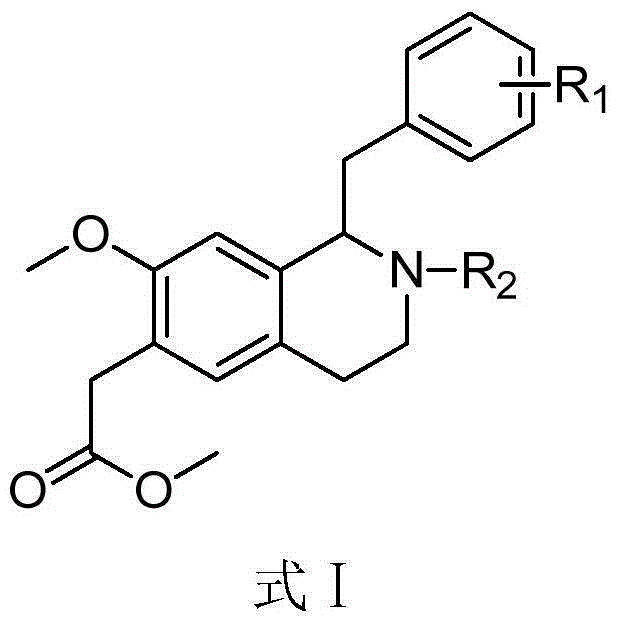
1-benzyl-6-carbomethoxy methyl tetrahydroisoquinoline derivative, preparation method and application in resisting aggregation of thrombocyte
A technology of methoxycarbonylmethyltetrahydroisoquinoline and methoxycarbonylmethyl, which is applied in the field of pharmaceutical synthesis and achieves the effects of correct structure, simple and easy preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0041] Example 12 Preparation of-[(1,1'-biphenyl)-4-yl]-N-(4-methoxyphenethyl)acetamide (Compound 9)
[0042] Weigh 1 mmol p-methoxyphenethylamine hydrochloride and 1.0-1.2 mmol felbinac, in a 25 mL single-neck round bottom flask, dissolve the raw material in 5 mL acetonitrile, add 4.2 mmol triethylamine, and stir magnetically in a water bath at 25°C. After 5 minutes, 0.4930 g (1.3 mmol) of peptide coupling agent HBTU was added, and the reaction was completed in 15-60 minutes. The acetonitrile was removed by rotary evaporation under reduced pressure to obtain a yellow solid, which was dissolved in 50 mL of dichloromethane, washed with deionized water (50 mL×3), and washed with 50 mL of saturated brine. The dichloromethane layer was dried with anhydrous sodium sulfate, filtered with suction, and rotated under reduced pressure. The solvent was evaporated to obtain a white solid. Column chromatography separation (dichloromethane: petroleum ether: ethyl acetate=100:25:4) to obtain 0...
Embodiment 22
[0043] Example 22-[(1,1'-Biphenyl)-4-yl]-N-(3-phenylmercaptomethoxycarbonylmethyl-4-methoxyphenethyl)acetamide (Compound 10) preparation
[0044] (1) Preparation of methyl 2-phenylmercaptoacetate (Compound 7). Measure 2054 μL (20.0 mmol) of thiophenol in a 100 mL three-necked flask, add 30 mL of toluene, cut 0.5060 g (22.0 mmol) of sodium metal into small pieces and add to the bottle, reflux the reaction at 110° C., and monitor the progress of the reaction by TLC. After the reaction is complete, 20.0-30.0 mmol of methyl chloroacetate are added dropwise with a constant pressure dropping funnel, and the reaction is monitored by TLC. The reaction is complete after 1 to 2 hours. 70 mL of ethyl acetate and 100 mL of deionized water were added to wash 3 times, the organic phase was dried with anhydrous sodium sulfate, filtered with suction, and the solvent was removed by rotary evaporation under reduced pressure to obtain a pale yellow oily liquid 1. Column chromatography separation ...
Embodiment 32
[0047] Example 32 Preparation of-[(1,1'-biphenyl)-4-yl]-N-(3-methoxycarbonylmethyl-4-methoxyphenethyl)acetamide (Compound 11)
[0048] Weigh 3.9813 g (7.6 mmol) of the compound into a 250 mL single-necked flask, and dissolve 80 mL of ethanol. The Raney nickel was washed with ethanol to remove water, 11.4 g of Raney nickel was added, and hydrogen was replaced 3 times. Under hydrogen, the reaction was completed under a water bath at 25°C and magnetically vigorously stirred for 2 to 4 hours. The reaction solution was suction filtered with diatomaceous earth, the filter cake was washed with dichloromethane, and the filtrate was rotary evaporated under reduced pressure to remove the solvent to obtain 3.0500 g of white solid with a yield of 97%. 1 HNMR(CDCl 3 ,400MHz)δ:7.59(d,J=7.2Hz,2H,H-2”,6”),7.55(d,J=8.0Hz,2H,H-4,8),7.44(dd,J 1 =7.2Hz,J 2 =7.2Hz,2H,H-3”,5”), 7.35(t,1H,J=7.2Hz,1H,H-4”), 7.26(d,J=8.0Hz,2H,H-5,7 ), 6.90(d,J=1.4Hz,H-4'),6.89(dd,1H,J 1 =1.4Hz,J 2 =8.0Hz,H-8'),6.68(d,J=8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com