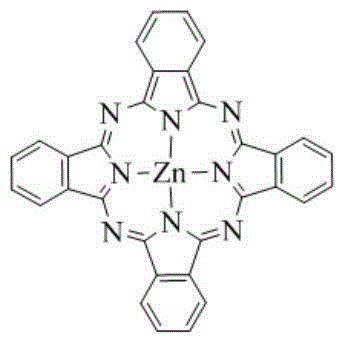
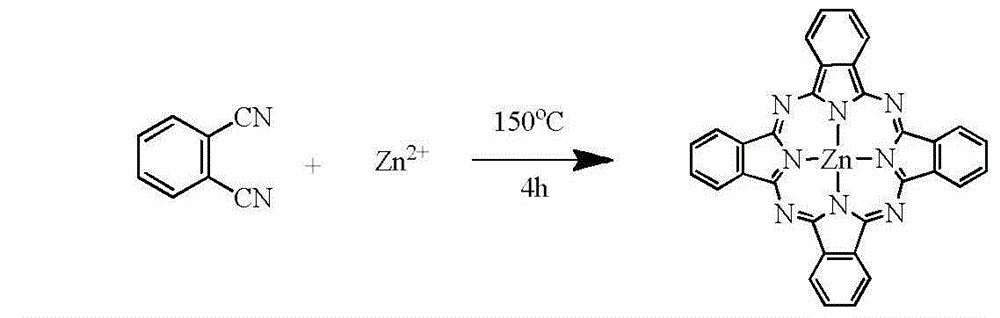Synthetic method of unsubstituted zinc phthalocyanine
A synthesis method and technology of zinc phthalocyanine, applied in the field of synthesizing unsubstituted zinc phthalocyanine, can solve the problems of complex purification method, long reaction time, high reaction temperature, etc., and achieve the effects of low production cost, simple synthesis method and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 The synthetic method of unsubstituted zinc phthalocyanine
[0019] Add 0.878g (4.0mmol) of zinc acetate dihydrate and 2.05g (16.0mmol) of phthalonitrile to 30ml of DMF solvent in sequence, and react in a hydrothermal reaction kettle at 150°C for 4h, stop the reaction and cool to At room temperature, the color of the solution was bright blue, and 300ml of ether was added. That is, a large amount of solid precipitated out and was suction filtered. The precipitate was repeatedly washed with dichloromethane and filtered with suction to obtain a large amount of purple-black solid, which was dried in vacuo to obtain 1.78 g of solid powder with a yield of 77%.
[0020] Elemental analysis (C 32 h 16 N 8 Zn), measured value (theoretical value) %: C66.82 (66.51), H2.69 (2.79), N19.14 (19.39).
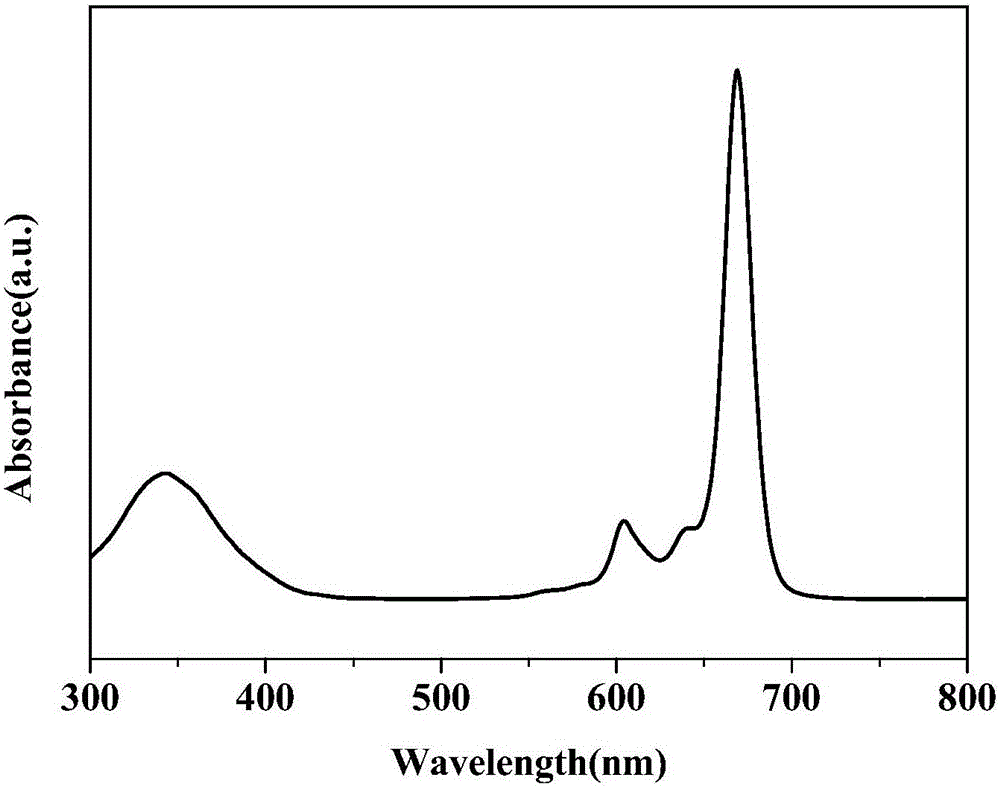
[0021] figure 1 is the UV-Vis absorption spectrum of unsubstituted zinc phthalocyanine in DMF solvent. It can be seen from the figure that 600-800nm is the Q-band cha...
Embodiment 2
[0022] The synthetic method of embodiment 2 unsubstituted zinc phthalocyanines
[0023] Add 0.878g (4.0mmol) of zinc acetate dihydrate and 2.05g (16.0mmol) of phthalonitrile to 30ml of DMF solvent in sequence, and react in a hydrothermal reaction kettle at 150°C for 4h, stop the reaction and cool to At room temperature, the color of the solution was bright blue, and 300ml of acetone was added. That is, a large amount of solid precipitated out and was suction filtered. The precipitate was repeatedly washed with dichloromethane and filtered with suction to obtain a large amount of purple-black solid, which was dried in vacuo to obtain 1.6 g of solid powder with a yield of 69%.
Embodiment 3
[0024] The synthetic method of embodiment 3 unsubstituted zinc phthalocyanines
[0025] Add 0.878g (4.0mmol) of zinc acetate dihydrate and 2.05g (16.0mmol) of phthalonitrile to 30ml of DMF solvent in sequence, and react in a hydrothermal reaction kettle at 150°C for 4h, stop the reaction and cool to At room temperature, the color of the solution was bright blue, and 300ml of ethyl acetate was added. That is, a large amount of solid precipitated out and was suction filtered. The precipitate was repeatedly washed with dichloromethane and filtered with suction to obtain a large amount of purple-black solid, which was dried in vacuo to obtain 1.55 g of solid powder with a yield of 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com