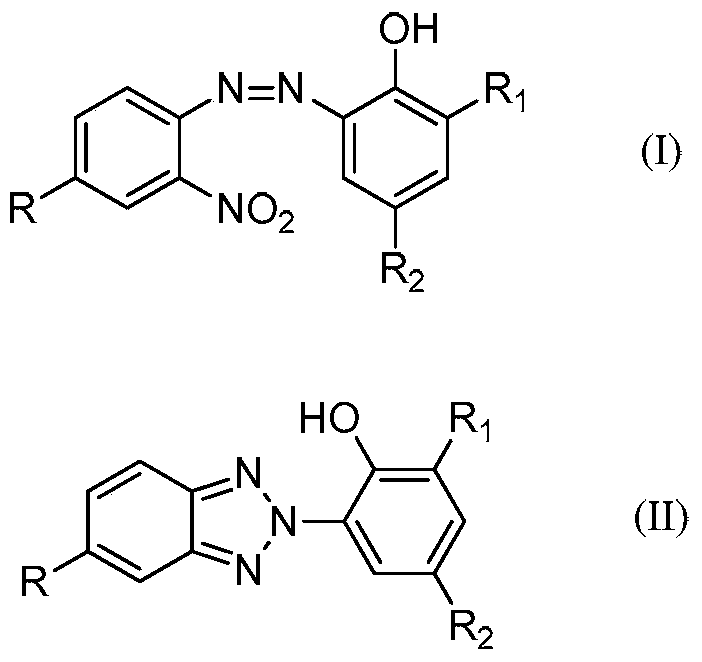
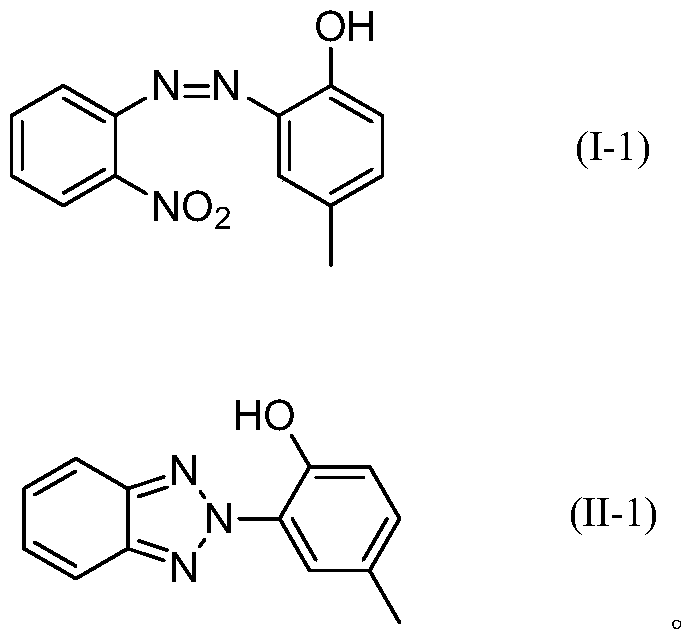
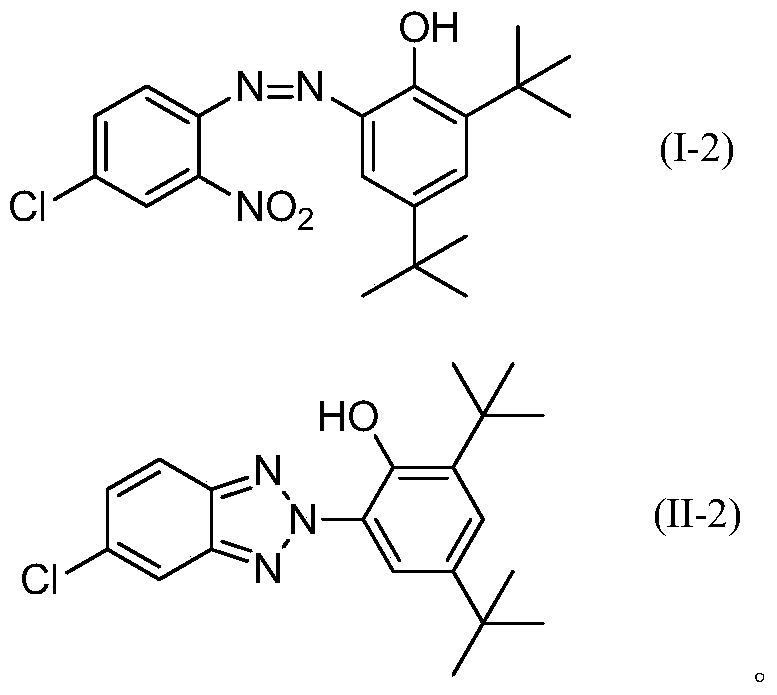
A kind of synthetic method of benzotriazole compound
A technology of benzotriazoles and a synthetic method, applied in the field of synthesizing benzotriazoles, can solve the problems such as failure to avoid the use of lye, increase production cost, difficulty in post-processing, and achieve simplified post-processing process, Easy to recycle, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of hydrogenation-solid base bifunctional catalyst (equal volume impregnation method):
[0030] The aqueous solution of platinum chloride and magnesium nitrate hexahydrate and the mixed carrier were impregnated in equal volumes for 12 hours to obtain a catalyst precursor (i.e. the carrier loaded with platinum chloride and magnesium nitrate hexahydrate). The mixed carrier was composed of Hβ molecular sieve and active oxidation Aluminum (the mass ratio of Hβ molecular sieve to activated alumina is 1:1), dried at 100°C for 24h, then calcined at 500°C for 4h; washed repeatedly with deionized water until the washing liquid no longer contained chloride ions, and then Dry at 100°C for 24 hours to obtain an oxidative hydrogenation-solid base dual-function catalyst; reduce at 350°C for 1 hour under 3MPa hydrogen pressure to obtain a hydrogenation-solid base dual-function catalyst. According to ICP-AES analysis, the mass content of hydrogenation active metal element pl...
Embodiment 2
[0033] A kind of synthetic method of benzotriazole compound (II-1), comprises the steps: the hydrogenation-solid base bifunctional catalyst that 40mL (15.23g) embodiment 1 prepares is packed in single tubular fixed-bed reactor , the inner diameter of the reactor is 14mm, and the pipe length is 650mm; the compound (purity is 98.43%) shown in the formula (I-1) is dissolved in 1,4-dioxane to obtain the compound shown in the formula (I-1) The mass concentration of the compound is 25% solution, the volume space velocity is 300h -1 The single-tubular fixed-bed reactor is continuously fed into the single-tubular fixed-bed reactor under certain conditions, and hydrogen is fed in, and heated so that the hydrogen pressure in the single-tubular fixed-bed reactor is 0.1MPa and the temperature is 150°C; the reaction liquid continues to flow from the single-tubular fixed-bed The lower end of the reactor flows out, and the reaction solution flowing out is cooled to room temperature, gas-liqu...
Embodiment 3
[0038] Preparation of hydrogenation-solid alkali bifunctional catalyst (secondary impregnation method):
[0039] The nickel nitrate aqueous solution, the potassium nitrate aqueous solution and the mixed carrier were impregnated by the second impregnation method, each time for 12 hours, a total of 2 times, to obtain the catalyst precursor (i.e. the carrier loaded with nickel nitrate and potassium nitrate), and the mixed carrier was made of HZSM- Composed of 5 molecular sieves and activated alumina, (the mass ratio of HZSM-5 molecular sieves to activated alumina is 9:1) dried at 130°C for 12 hours, then roasted at 500°C for 4 hours, washed repeatedly with deionized water into the washing liquid It no longer contains nitrate ions, and then dried at 130°C for 12 hours to obtain an oxidized hydrogenation-solid base dual-function catalyst; at 360°C and 0.1MPa hydrogen pressure for 5 hours, a hydrogenation-solid base dual-function catalyst was obtained. According to ICP-AES analysis,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com