Preparation method for three-element copper-iron-sulfur (CuFeS2) fluorescent quantum dot with magnetic property by aqueous-phase synthesis
A technology of fluorescent quantum dots and ternary copper, applied in chemical instruments and methods, iron sulfide, nano optics, etc., can solve the problems of limited application, poor dispersion, large size of quantum dots, etc., and achieve low raw material cost and good dispersion , The effect of simple production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
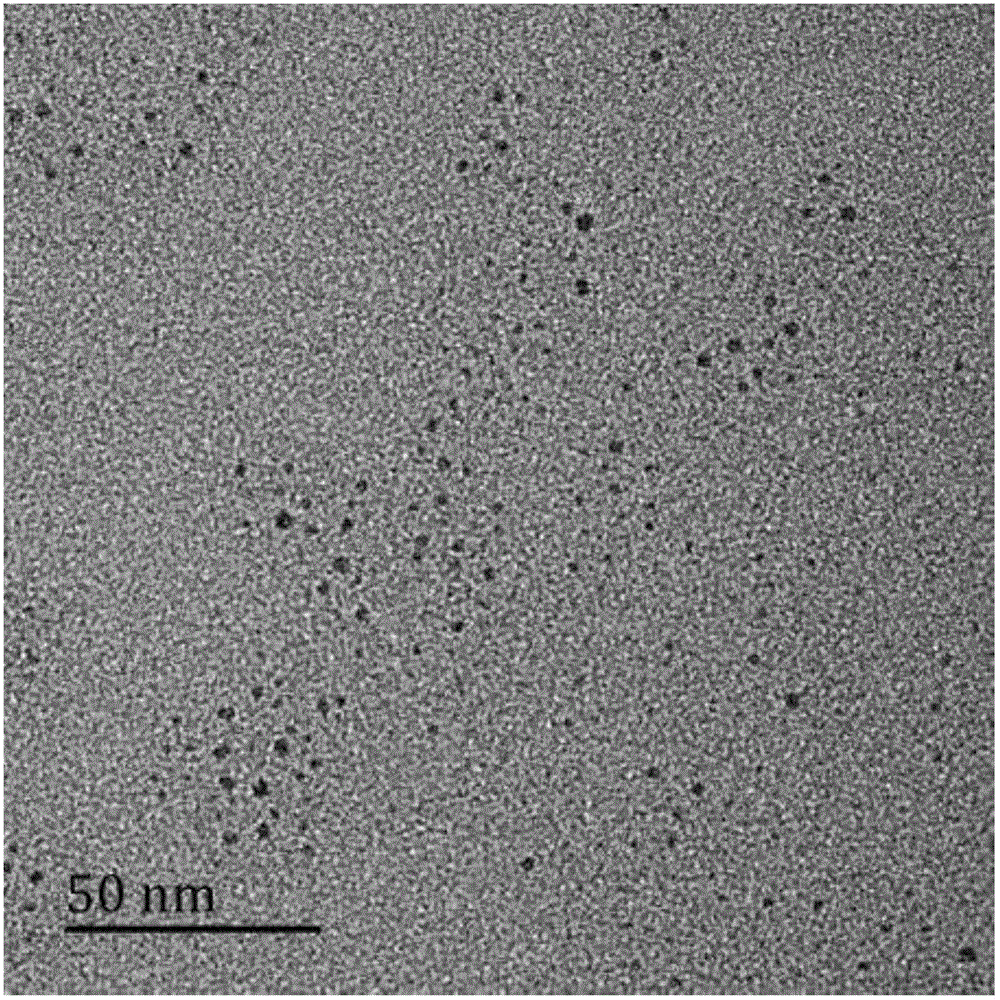
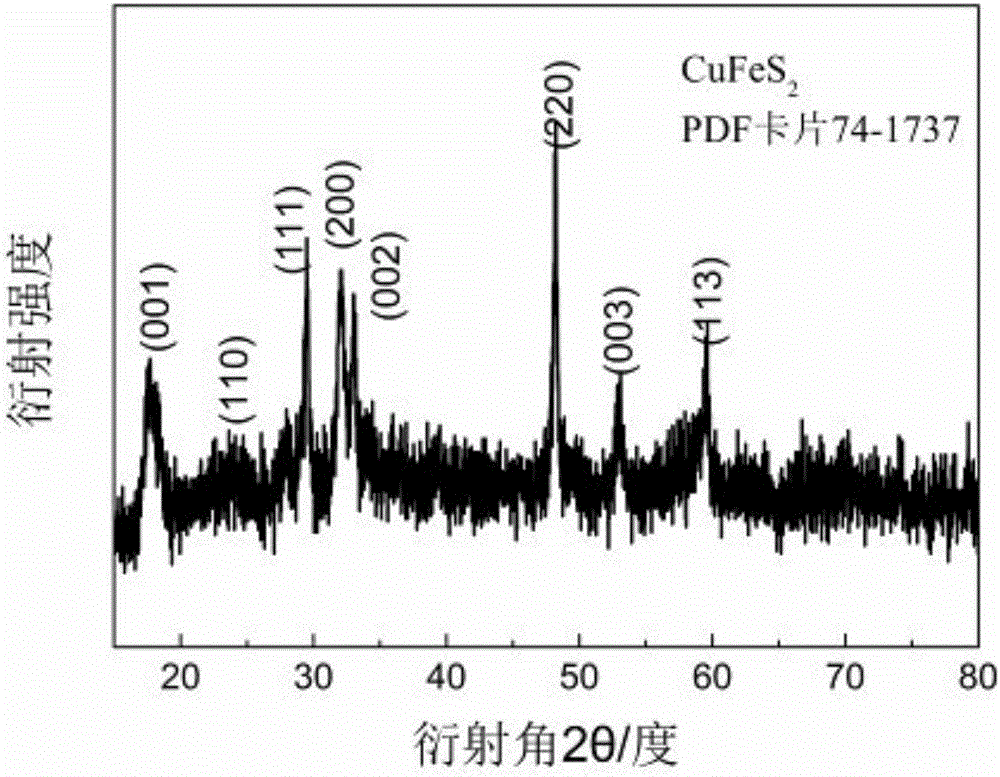
[0029] 0.0301g of thioacetamide (TAA) was dissolved in 20mL of diethylene glycol (DEG) to prepare an anion solution. 0.0966g of copper nitrate and 0.1616g of ferric nitrate were dissolved in 20mLDEG to prepare a cationic solution for later use. Add 20 mL of the cation solution above into a 100 mL three-necked flask, and raise the temperature of the oil bath to 160° C. to reflux. The anionic solution was quickly injected into the above solution and refluxed for 60 minutes. After the reaction, the solution was cooled to room temperature. Acetone was added to the obtained product until precipitation occurred, and then centrifuged in a centrifuge at 6000 rpm for 8 minutes. The centrifuged precipitate was redispersed in acetone and then centrifuged at the same speed to obtain the precipitate, and the product after washing three times was dried at room temperature to obtain CuFeS 2 Fluorescent quantum dots.
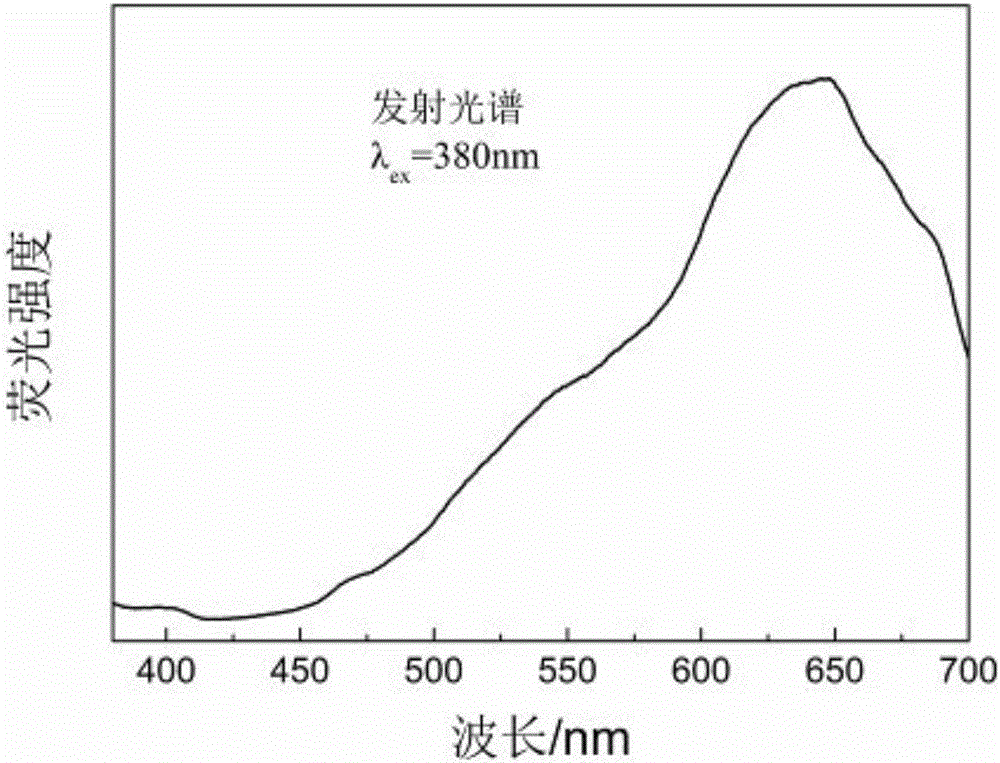
[0030] figure 1 CuFeS 2 The emission spectrum of quantum dots shows...
Embodiment 2
[0032]0.0301g of thioacetamide (TAA) was dissolved in 20mL of ethylene glycol (EG) to prepare an anion solution. 0.0966g of copper nitrate and 0.1616g of ferric nitrate were dissolved in 20mLEG to prepare a cationic solution for future use. Add 20 mL of the cation solution above into a 100 mL three-necked flask, and raise the temperature of the oil bath to 120° C. to reflux. The anionic solution was quickly injected into the above solution and refluxed for 45 minutes. After the reaction, the solution was cooled to room temperature. Acetone was added to the obtained product until precipitation occurred, and then centrifuged in a centrifuge at 6000 rpm for 8 minutes. The centrifuged precipitate was redispersed in acetone and then centrifuged at the same speed to obtain the precipitate, and the product after washing three times was dried at room temperature to obtain CuFeS 2 quantum dots.
[0033] Figure 5 CuFeS 2 The emission spectrum of quantum dots shows that CuFeS 2 Q...
Embodiment 3
[0035] 0.0301g of thioacetamide (TAA) was dissolved in 20mL of water to prepare an anion solution. 0.0483g of copper nitrate and 0.2424g of ferric nitrate were dissolved in 20mL of water to prepare a cationic solution for later use. Add 20 mL of the cation solution above into a 100 mL three-necked flask, and raise the temperature of the oil bath to 90° C. to reflux. The anionic solution was quickly injected into the above solution and refluxed for 75 minutes. After the reaction, the solution was cooled to room temperature. Acetone was added to the obtained product until precipitation occurred, and then centrifuged in a centrifuge at 6000 rpm for 8 minutes. The centrifuged precipitate was redispersed in acetone and then centrifuged at the same speed to obtain the precipitate, and the product after washing three times was dried at room temperature to obtain CuFeS 2 quantum dots.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com