Method for efficient expression and secretion of transpeptidase Sortase A
A technology of transpeptidase and expression vector, which is applied in the field of genetic engineering, can solve the problems of the extracellular secretion of the added concentration and the added time of the target protein, inconsistent effects, inconsistent effects, etc., to shorten the fermentation time, save production costs, and realize industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Extracellular production of SortaseA with Escherichia coli E.coliBL21 (DE3)
[0026] The construction method of the recombinant Escherichia coli used in the present invention to produce Sortase A is: take the genome of S.aureus as a template, amplify with srt-F and srt-R (Table 1) primers to remove the N-terminal signal peptide (59 amino acids) The srt gene and the T vector were ligated and transformed into E.coliJM109. After the correctness was verified by sequencing, they were subcloned into plasmids pET22b and pET20b and transformed into E. coli expression host E.coliBL21(DE3). The recombinant Escherichia coli (pET22b-srt and pET20b-srt) were induced and expressed in 250mL containing 25mL medium, the induction temperature was 30°C, the rotation speed was 250rpm, and the final concentrations of the inducer IPTG were 1mM / L and 0.4mM respectively / L. Fermentation results showed that active Sortase A could be detected in the fermentation broth of the strain us...
Embodiment 2
[0027] The comparative screening of embodiment 2 different additives
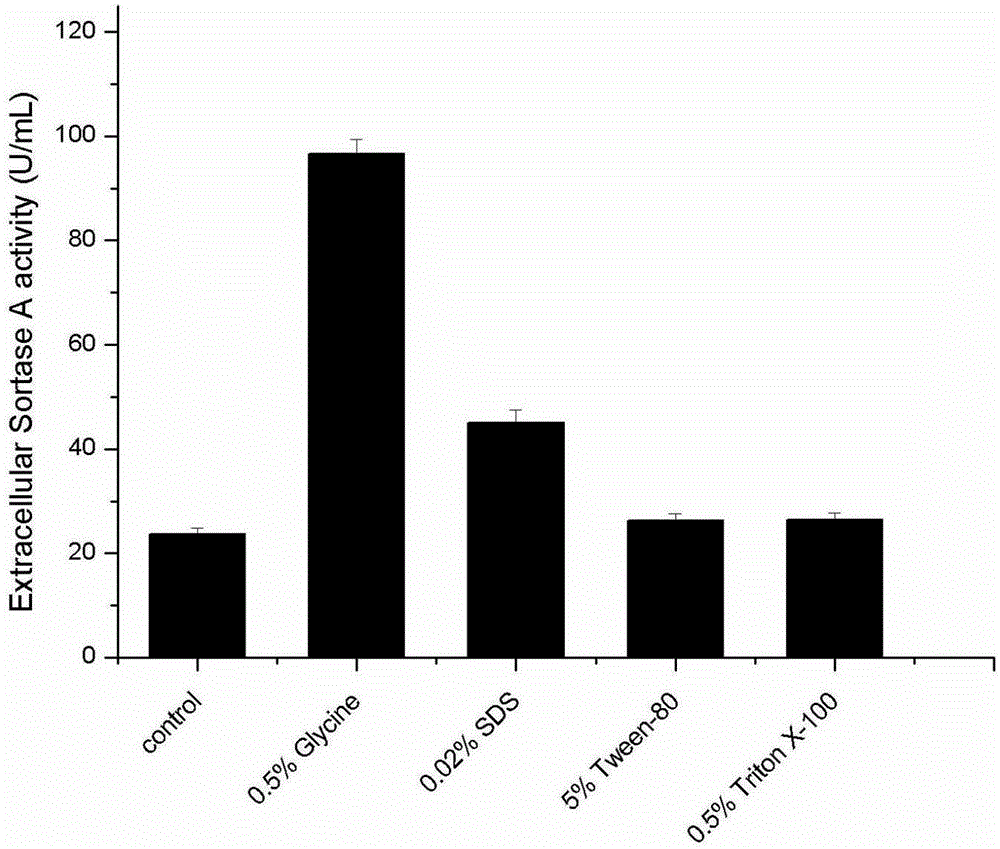
[0028]In order to evaluate the effect of different additives on the extracellular production of SortaseA in Escherichia coli, the effect of different additives on the extracellular SortaseA enzyme activity was screened first. Additives were added together with IPTG during induction. Or the cell wall all has a destructive effect, considering the cost and toxicity of the additive, SDS, glycine, Tween 80, Triton X-100 are selected as the additive of the preliminary test, the result ( figure 1 ) shows that adding 0.5% (m / v) glycine during induction has obvious promotion effect on extracellular enzyme activity, and adding 5% Tween 80 or 0.5% Triton 100 promotes the extracellular enzyme activity of SortaseA The effect is almost 0. Therefore, glycine was selected as the additive for the next experiment, and other additives were not studied here.
Embodiment 3
[0029] The screening of embodiment 3 different concentrations of glycine
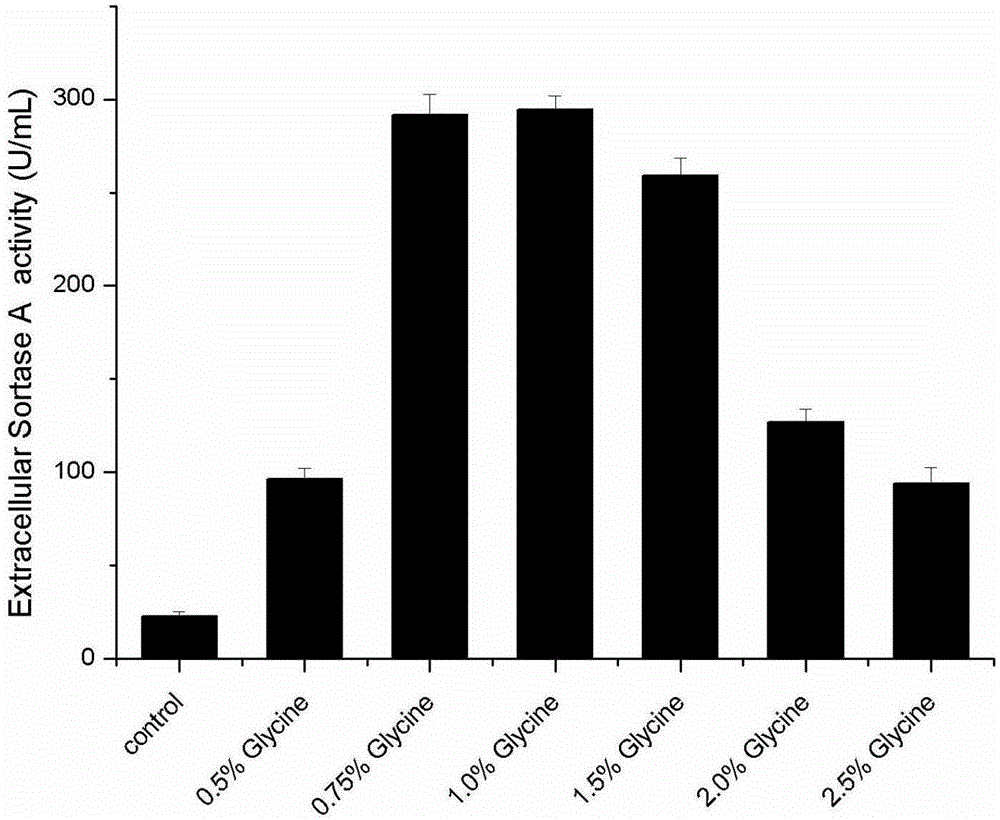
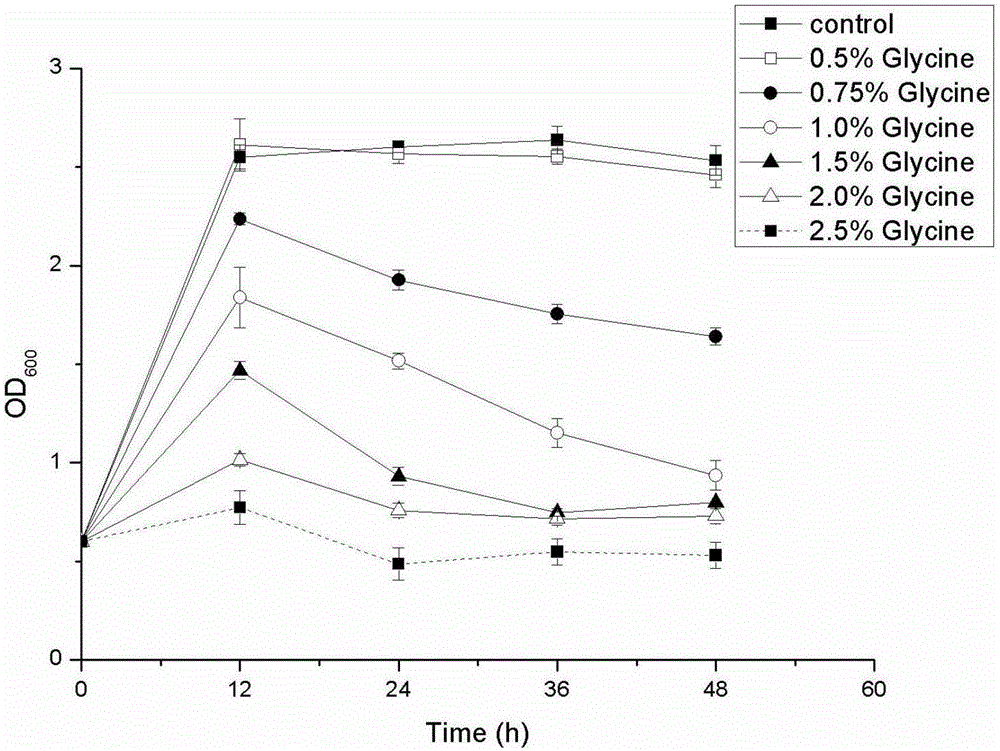
[0030] In order to compare the influence of adding different final concentrations (m / v) glycine on E. coli extracellular production of SortaseA when inducing, selected 0.5%, 0.75%, 1.0%, 1.5%, 2.0%, 2.5% six kinds of addition levels, the result ( figure 2 ) shows that 0.75% and 1.0% are more obvious to the extracellular production promotion effect of SortaseA, but considering additive cost and thalline growth ( image 3 ), the added concentration of 0.75% is preferred.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com