Chromium metal organic framework catalytic material and preparation method thereof
A technology of organic framework and catalytic materials, which is applied in the field of organic chemistry and material chemistry, and in the field of inorganic chemistry, can solve the problems of complex synthesis process, increased industrial cost, unfavorable energy saving and environmental protection, etc., and achieve good stability, high activity and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
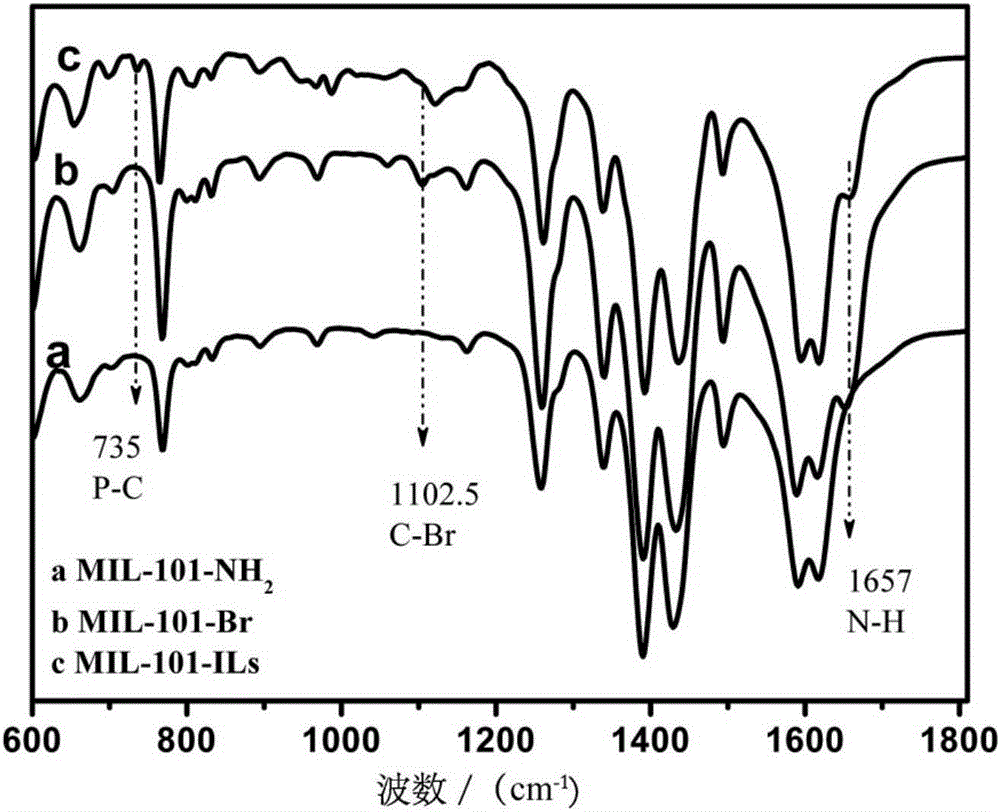
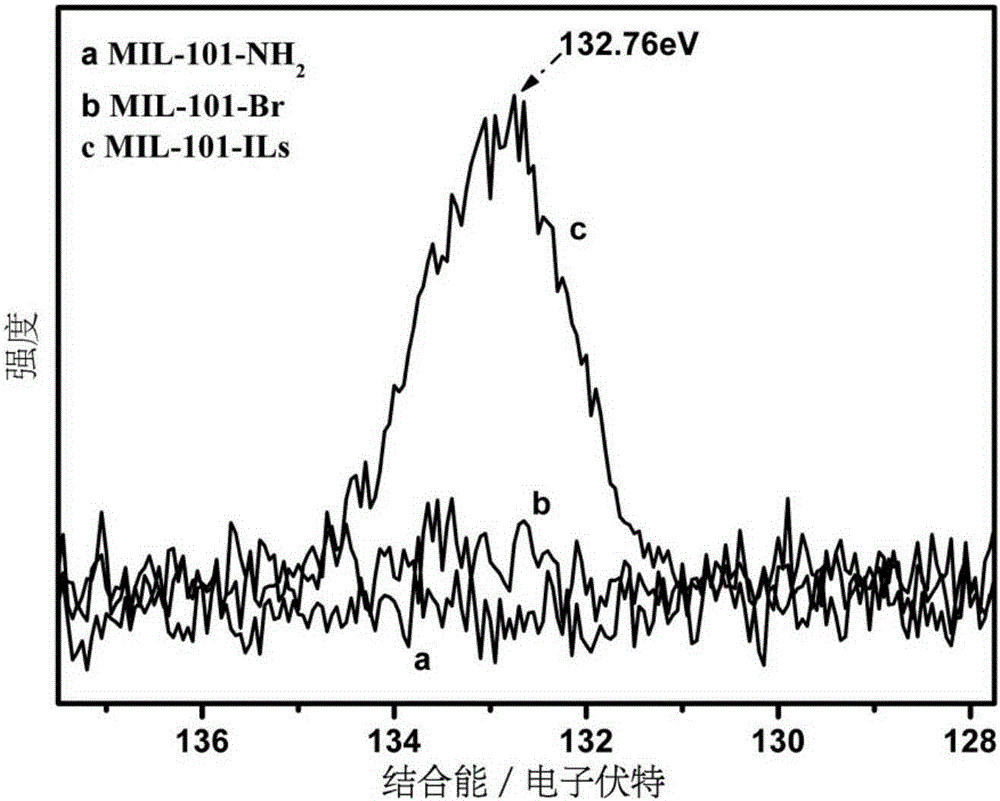
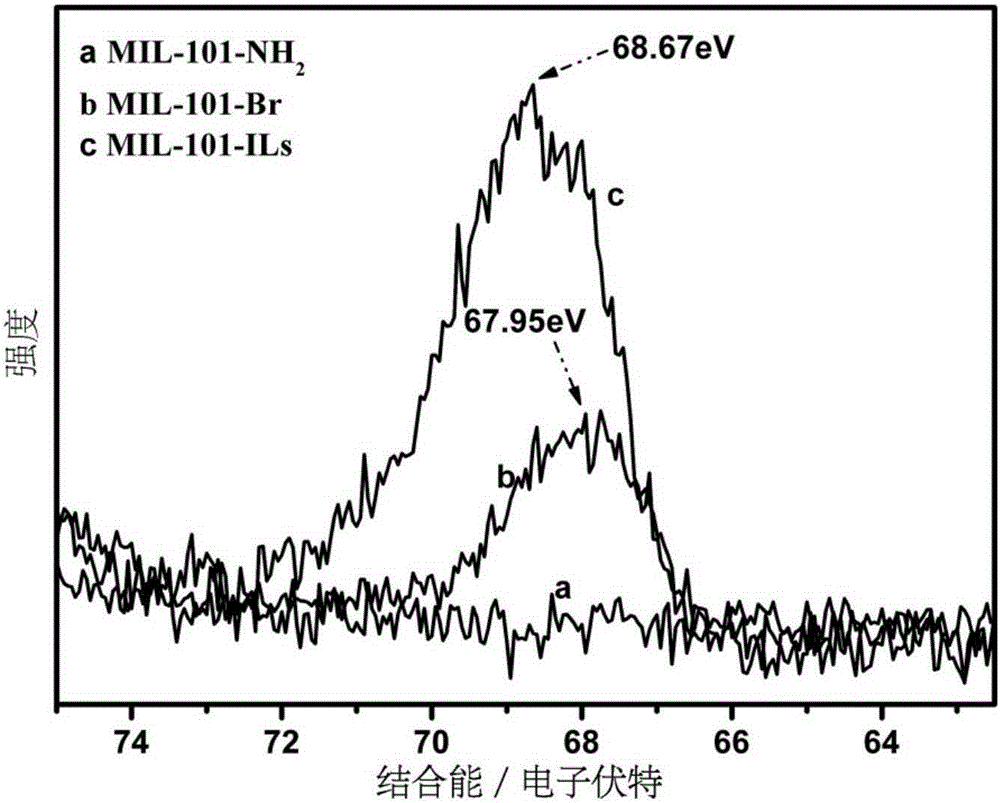
[0028] (1) Add 0.001mol chromium chloride, 0.00075mol 2-nitroterephthalic acid and 0.25mol deionized water 1 into the hydrothermal reaction kettle, and react at 180°C for 3 days; Washing with water and absolute ethanol for 3 times respectively; using 0.5mol deionized water 2 and 0.5mol absolute ethanol 1 as solvents respectively, activated in a microwave reactor at 130°C for 3 hours; Vacuum drying at 100°C for 12 hours at 133Pa to obtain MIL-101-NO 2 ; 0.001mol of the MIL-101-NO 2 Add 0.001mol stannous chloride monohydrate into 0.2mol absolute ethanol 2, react at 70°C for 10 hours, cool to room temperature, centrifuge, wash 3 times with concentrated hydrochloric acid, wash 3 times with deionized water, and wash 3 times with absolute ethanol times; vacuum drying at 100°C for 12 hours under a vacuum degree of 133Pa to obtain MIL-101-NH 2 ;
[0029] (2) At room temperature, 0.001mol of the MIL-101-NH 2 Disperse in a mixed solution of 0.1mol acetonitrile and 0.05molDMF; add 0....
Embodiment 2
[0039] (1) Add 0.001mol chromium chloride, 0.001mol 2-nitroterephthalic acid and 0.277mol deionized water 1 into the hydrothermal reaction kettle, and react at 180°C for 4 days; Wash with water and absolute ethanol for 3 times respectively; use 0.6mol deionized water and 0.6mol absolute ethanol as solvents respectively, and activate at 140°C for 2 hours in a microwave reactor; Dry under vacuum at 110°C for 13 hours to obtain MIL-101-NO 2 ; 0.001mol of the MIL-101-NO 2 Add 0.0015mol stannous chloride monohydrate into 0.22mol absolute ethanol 2, react at 80°C for 10 hours, cool to room temperature, centrifuge, wash 4 times with concentrated hydrochloric acid, wash 4 times with deionized water, and wash 4 times with absolute ethanol times; vacuum drying at 110°C for 13 hours under a vacuum degree of 133Pa to obtain MIL-101-NH 2 ;
[0040] (2) At room temperature, 0.001mol of the MIL-101-NH 2 Disperse in a mixed solution of 0.12mol acetonitrile and 0.05molDMF; add 0.004mol1,2-...
Embodiment 3
[0044] (1) Add 0.001mol chromium chloride, 0.002mol 2-nitroterephthalic acid and 0.3mol deionized water 1 into the hydrothermal reaction kettle, and react at 180°C for 5 days; Wash with water and absolute ethanol for 4 times respectively; use 0.7mol deionized water and 0.7mol absolute ethanol as solvents respectively, and activate at 150°C for 1 hour in a microwave reactor; Dry under vacuum at 120°C for 13 hours to obtain MIL-101-NO 2 ; 0.001mol of the MIL-101-NO 2 Add 0.0017mol stannous chloride monohydrate into 0.27mol absolute ethanol 2, react at 80°C for 12 hours, cool to room temperature, centrifuge, wash 4 times with concentrated hydrochloric acid, wash 4 times with deionized water, and wash 4 times with absolute ethanol times; vacuum drying at 120°C for 14 hours under a vacuum degree of 133Pa to obtain MIL-101-NH 2 ;
[0045] (2) At room temperature, 0.001mol of the MIL-101-NH 2 Disperse in a mixed solution of 0.14mol acetonitrile and 0.06molDMF; add 0.005mol1,2-dib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com