Preparation method for 2-nitro-4-(trifluoromethyl)benzonitrile
A technology of trifluoromethylbenzonitrile and chlorotrifluorotoluene is applied in the field of preparation of 2-nitro-4-trifluoromethylbenzonitrile and can solve the problem of 3-nitro-4-bromotrifluorotoluene Expensive, difficult to obtain, complex process and other problems, to achieve the effect of increasing selectivity and conversion rate, easy availability of raw materials, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
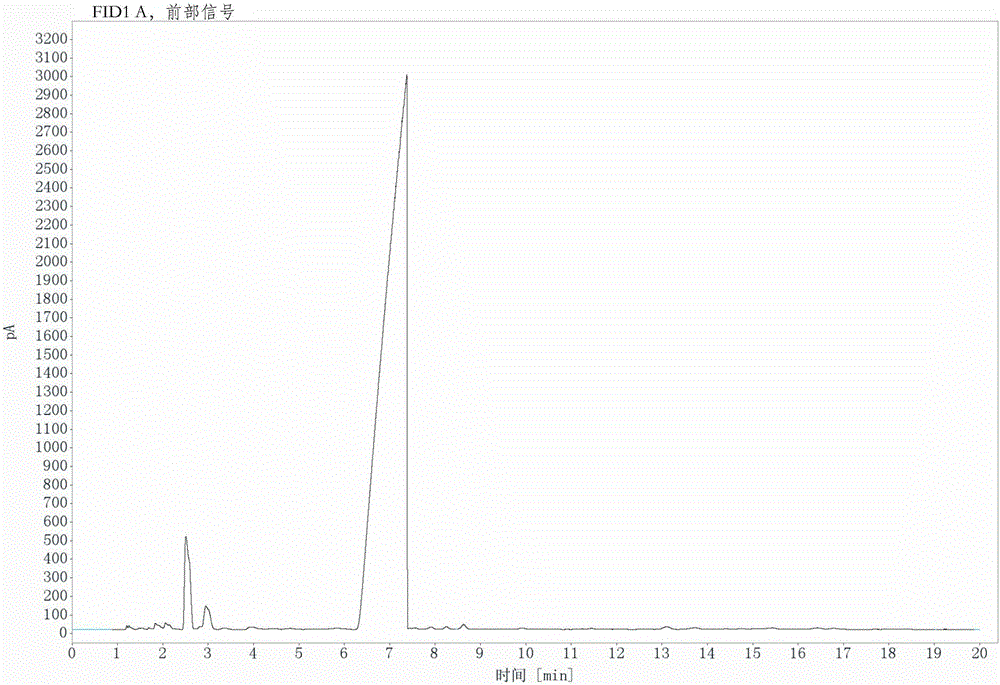
[0026] Under mechanical stirring and nitrogen protection, 258.0 g (1 mol) of 3-nitro-4-chlorobenzotrifluoride, 53.9 g (1.1 mol) of sodium cyanide, 143.0 g (1 mol) of cuprous bromide, 2.2g (0.01mol) of 1-butyl-3-methylimidazolium bromide, 99.0g (1mol) of N-methylpyrrolidone as a solvent, heat up under mechanical stirring, keep at 185°C for 3 hours, then raise the temperature to 192°C for 5 hours, The reaction is over. The conversion rate is 96.46% and the selectivity is 95.14% as detected by GC, and the content of the product obtained by rectification can reach more than 99% as detected by GC.
[0027] Such as figure 1 As shown in the GC detection chart, it can be seen from the figure that the retention time is 2.51min and the peak area is 3.54, indicating that the content of the raw material 3-nitro-4-chlorotrifluoromethyl toluene is 3.54%, so its conversion rate is 96.46%, and the retention time The peak area at 7.37min was 91.77, indicating that the content of the product ...
Embodiment 2
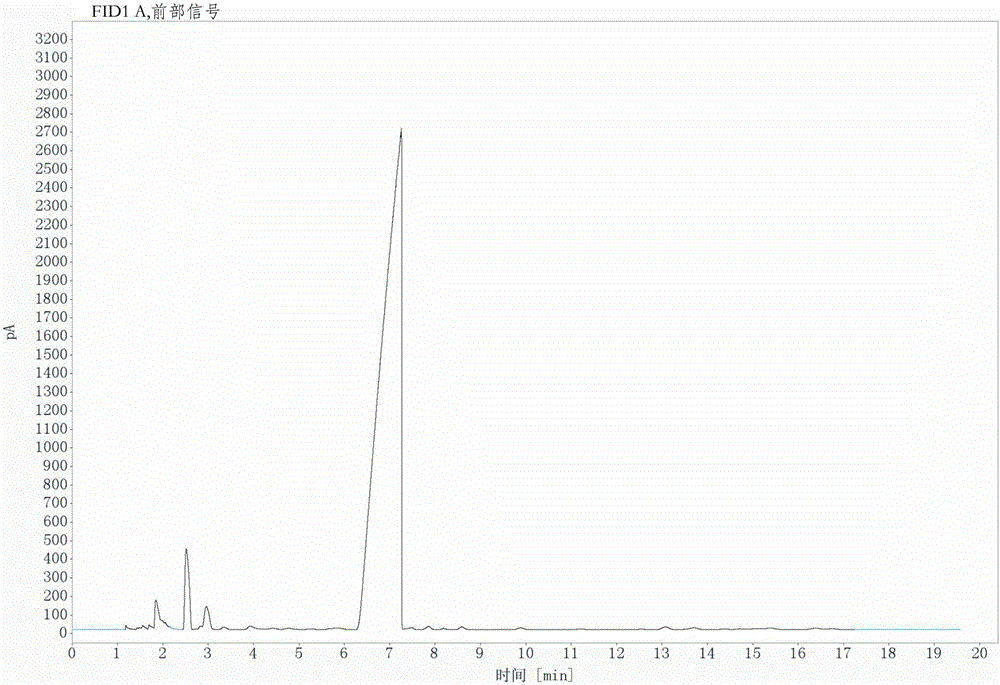
[0029] Under mechanical stirring and nitrogen protection, 258.0g (1mol) of 3-nitro-4-chlorobenzotrifluoride, 98.5g (1.1mol) of cuprous cyanide, 218.5g (1mol) of nickel bromide, 1.7g (0.01mol) of 1-butyl-3-methylimidazolium chloride salt, 99.0g (1mol) of N-methylpyrrolidone as a solvent, heat up under mechanical stirring, keep at 185°C for 3 hours, then raise the temperature to 193°C for 5 hours, The reaction is over. According to GC detection, the conversion rate is 96.73%, and the selectivity is 95.20%. The content of the product obtained through rectification can reach more than 99% through GC detection.
[0030] Such as figure 2 As shown in the GC detection chart, it can be seen from the figure that the retention time is 2.52min and the peak area is 3.27, indicating that the content of the raw material 3-nitro-4-chlorotrifluoromethyltoluene is 3.27%, so its conversion rate is 96.73%, and the retention The peak area at time 7.25min was 92.09, indicating that the content o...
Embodiment 3
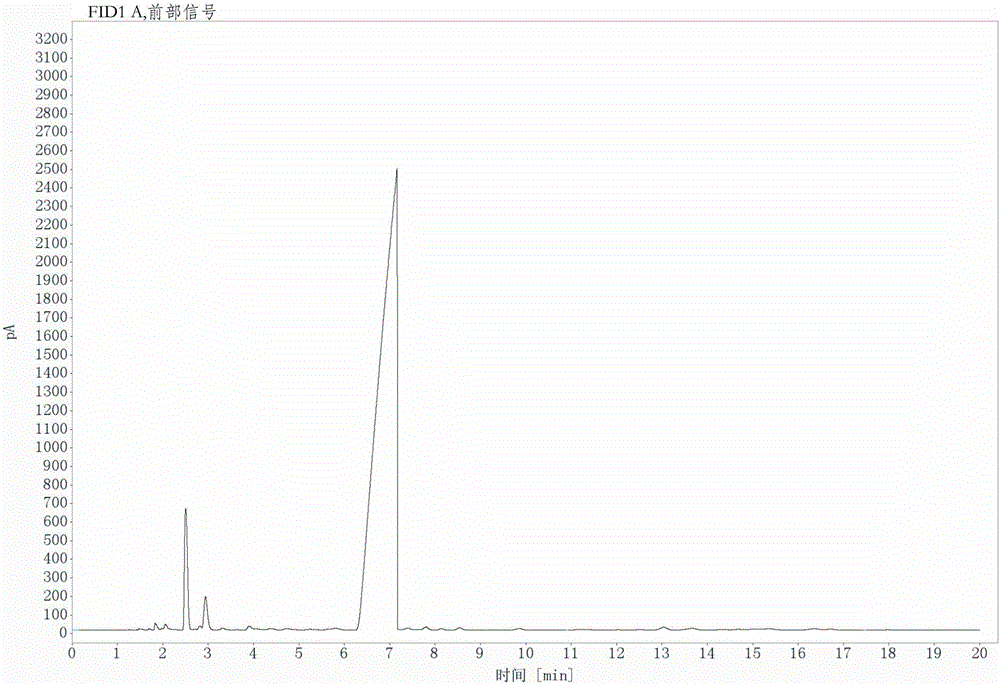
[0032] Under mechanical stirring and nitrogen protection, 258.0g (1mol) of 3-nitro-4-chlorobenzotrifluoride, 98.5g (1.1mol) of cuprous cyanide, and 143.0g (1mol) of cuprous bromide were successively added to the reactor , 2.1g (0.01mol) of 1-propyl-3-methylimidazolium bromide, 99.0g (1mol) of N-methylpyrrolidone as a solvent, heat up under mechanical stirring, keep warm at 185°C for 4 hours, then heat up to 195°C for 5 hours , the reaction ends. The conversion rate is 96.95% and the selectivity is 95.65% through GC detection, and the content of the product obtained through rectification can reach more than 99% through GC detection.
[0033] Such as image 3 As shown in the GC detection chart, it can be seen from the figure that the retention time is 2.51min and the peak area is 4.05, indicating that the raw material 3-nitro-4-chlorotrifluoromethyltoluene content is 3.05%, so its conversion rate is 96.95%, and the retention Time 7.16min peak area is 92.73, illustrates that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com