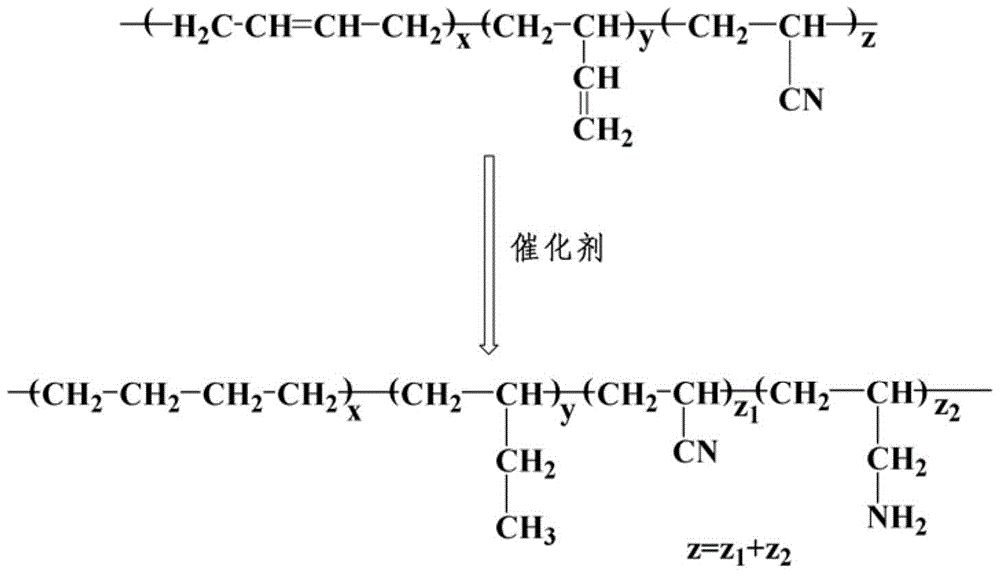
A kind of preparation method and obtained product of hydrophilic aminated hydrogenated nitrile rubber
A technology of aminated hydrogenated nitrile and nitrile rubber, which is applied in the field of organic polymer compounds to achieve the effect of broadening applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: preparation nanometer Rh metal catalyst
[0033] 1. Preparation of RhCl 3 ·3H 2 O aqueous solution
[0034] 263.5mg RhCl 3 ·3H 2 O salt was mixed with 40mL deionized water, and magnetically stirred at room temperature for 12h to obtain RhCl 3 ·3H 2 Aqueous solution of O precursor, that is, liquid A;
[0035] 2. Prepare the hydrazine hydrate solution of sodium hydroxide
[0036] Sodium hydroxide is dissolved in the hydrazine hydrate solution, and magnetically stirred evenly to obtain the hydrazine hydrate solution of sodium hydroxide, namely liquid B. The molar weight of sodium hydroxide is 12mmol, and the mol ratio of sodium hydroxide and hydrazine hydrate is 1:2;
[0037] 3. Mixing of two solutions
[0038] Liquid A was dropped into solution B at 60° C., magnetically stirred for 4 hours, the prepared product was subjected to centrifugal suction filtration, and dried to obtain a black powder, that is, nano-Rh catalyst.
Embodiment 2
[0039] Embodiment 2: the preparation of aminated hydrogenated nitrile rubber
[0040] 1. Mix the 60mg nanometer Rh metal catalyst prepared in Example 1 with nitrile rubber, and ultrasonically mix for 10 minutes; the mass ratio of nanometer Rh metal catalyst to nitrile rubber dry weight is 2%.
[0041] 2. Put the mixed liquid obtained in step 1 into the lining of the reactor, and carry out the first step of catalytic hydrogenation under the conditions of 80°C, 1000r / min, and 4MPa hydrogen pressure, and react for 5.5h;
[0042] 3. The first step catalytic hydrogenation reaction product is centrifuged to remove the black nano-metal Rh catalyst, and the mass fraction is added to the reactant to account for 1% RhCl (PPh 3 ) 3 The catalyst was reacted at 120° C. for 2 hours under 4 MPa hydrogen pressure, and the reaction product was dried: the drying temperature was 50° C., and the drying was done for 12 hours.
[0043] The route of aminated hydrogenated nitrile butadiene rubber p...
Embodiment 3
[0045] The preparation method is the same as in Example 2, except that the reaction times in step 2 are 1h, 2h, 3h, and 4h, respectively.
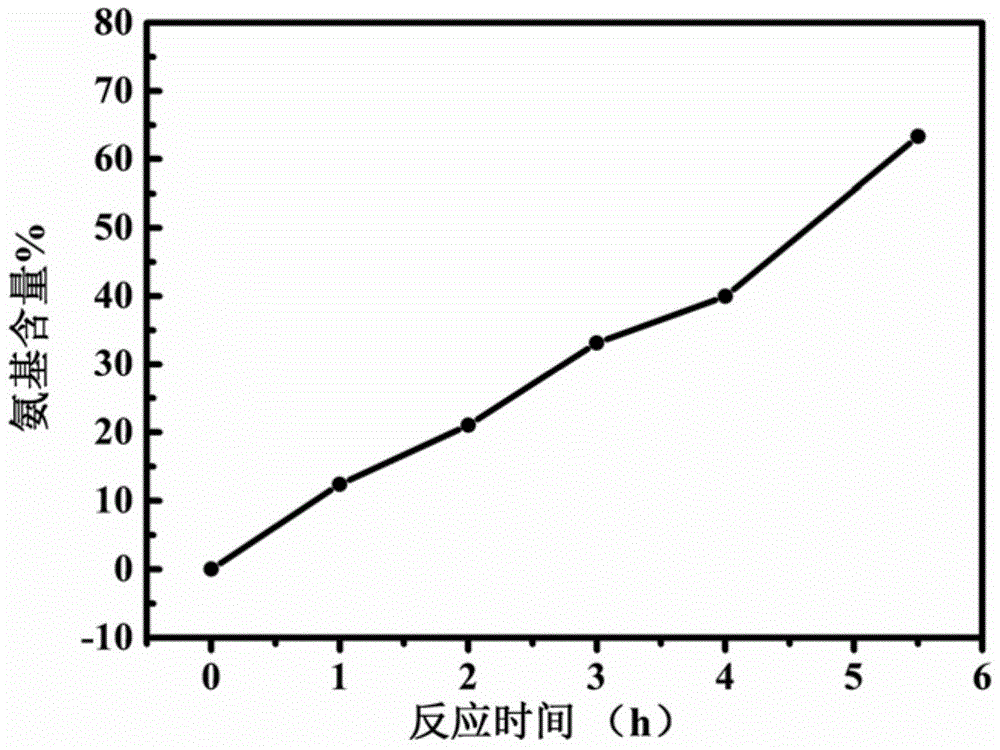
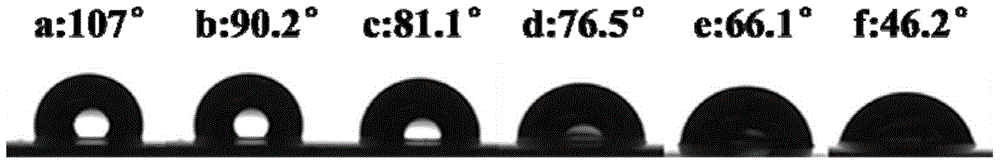
[0046] The content of amino groups is shown in Table 1, and the results of NMR characterization are shown in figure 2 . Carry out contact angle test to sample, test result is shown in Table 2 and image 3 . image 3 Among them, a~f correspond to amino content of 0%, 12.42%, 21.04%, 33.13%, 40%, 63.3%, respectively.
[0047] Table 1 Effect of reaction time on amino group content
[0048] Reaction time / h 1 2 3 4 5.5 Amino content% 12.42 21.04 33.13 40 63.3
[0049] It can be seen from Table 1 that by controlling the reaction time, aminated hydrogenated nitrile rubber with controllable amino content can be prepared, and the amino content gradually increases with the prolongation of the reaction time.
[0050] Table 2 Effect of amino group content on contact angle
[0051] Amino content% 0 12.42 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com