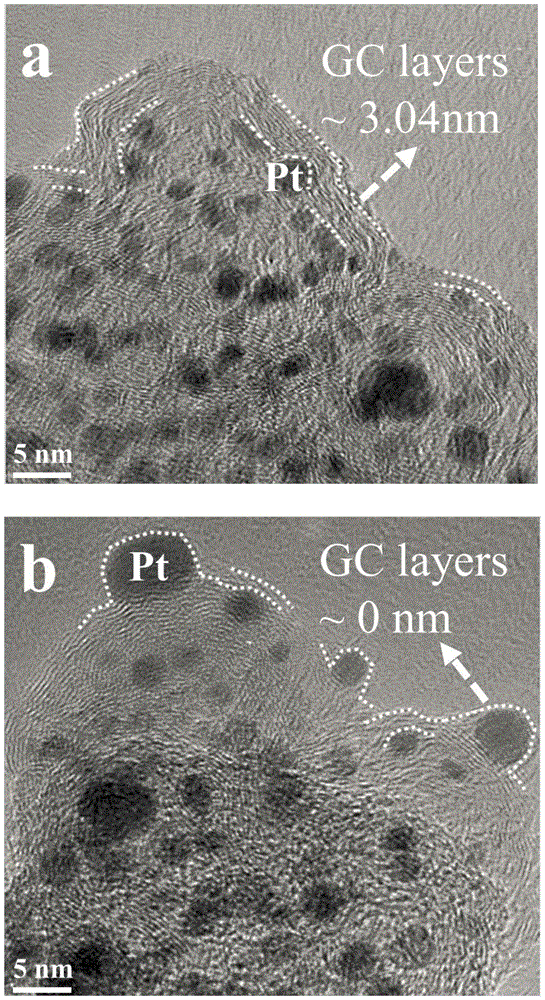
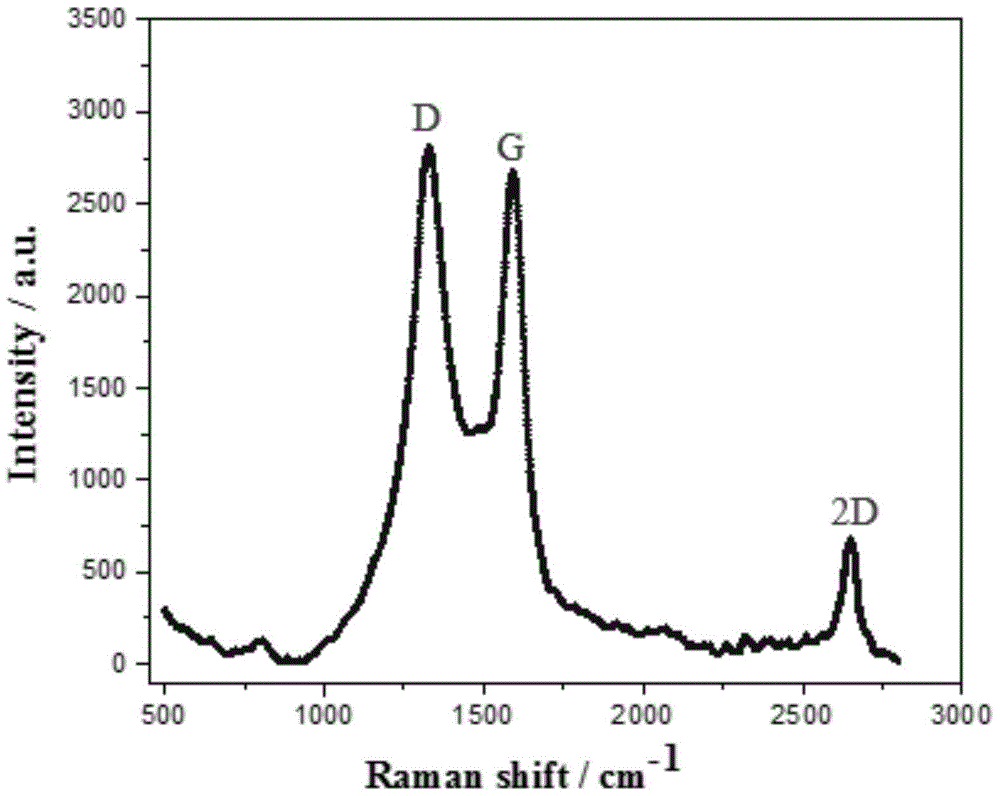
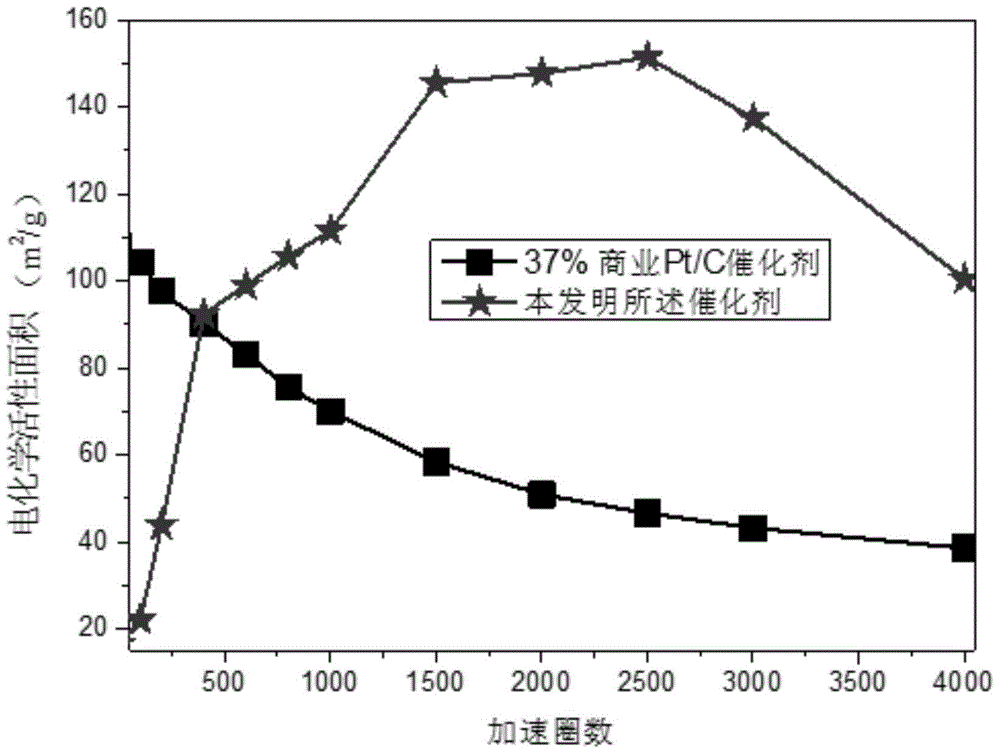
Fuel cell catalyst with nanographite carbon rivet structure and preparation method thereof
A nano-graphite and fuel cell technology, applied in fuel cells, battery electrodes, structural parts, etc., to achieve good stability, reduce migration and agglomeration, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 50mg, 37% Pt / C catalyst in a small porcelain boat, shake the small porcelain boat, so that the sample is evenly spread in the small porcelain boat, slowly put the small porcelain boat containing the sample into the quartz tube resistor central position of the furnace;
[0035] Seal the experimental device with silica gel, check the airtightness to ensure good;
[0036] Infuse high-purity argon for 1 hour at room temperature to remove the air in the device;
[0037] In a high-purity argon atmosphere, heat up to 80°C at a rate of 5°C / min, hold for 2 hours, and remove the moisture in the sample;
[0038] Raise the temperature to 700°C at 10°C / min. After the temperature is constant, turn off the high-purity argon gas, and feed C at a flow rate of 15ml / min. 2 h 2 Gas, time 10s;
[0039] After the reaction was completed, the acetylene gas valve was slowly closed and high-purity argon gas was introduced, and the temperature was kept for 30 minutes until the temperature...
Embodiment 2
[0062] Weigh 50mg, 37% Ru / C catalyst in a small porcelain boat, shake the small porcelain boat, so that the sample is evenly spread in the small porcelain boat, slowly put the small porcelain boat with the sample into the quartz tube resistor central position of the furnace;
[0063] Seal the experimental device with silica gel, check the airtightness to ensure good;
[0064] Inject high-purity nitrogen gas at room temperature for 1 hour to remove the air in the device;
[0065] In a high-purity nitrogen atmosphere, heat up to 100°C at a rate of 5°C / min and hold for 2 hours to remove the moisture in the sample;
[0066] Raise the temperature to 750°C at 10°C / min. After the temperature is constant, turn off the high-purity nitrogen gas and feed in methane gas at 8ml / min for 30s;
[0067] After the reaction was completed, the methane gas valve was slowly closed and high-purity nitrogen gas was introduced, and the temperature was kept for 45 minutes until the temperature droppe...
Embodiment 3
[0072] Weigh 50mg, 37% Pd / C catalyst in a small porcelain boat, shake the small porcelain boat, so that the sample is evenly spread in the small porcelain boat, slowly put the small porcelain boat containing the sample into the quartz tube resistor central position of the furnace;
[0073] Seal the experimental device with silica gel, check the airtightness to ensure good;
[0074] Infuse high-purity argon for 1 hour at room temperature to remove the air in the device;
[0075] In a high-purity argon atmosphere, heat up to 120°C at a rate of 8°C / min, hold for 1 hour, and remove the moisture in the sample;
[0076] Raise the temperature to 600°C at 10°C / min. After the temperature is constant, turn off the high-purity argon gas and feed in ethane gas at a flow rate of 10ml / min for 20s;
[0077] After the reaction was completed, the ethane gas valve was slowly closed and high-purity argon gas was introduced, and the temperature was kept for 1 h until the temperature dropped to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com