Method for preventing and/or treating infections, colonisations, or illnesses related to staphylococcus aureus, pseudomonas aeruginosa, streptococcus pyogenes, enterococcus faecium, enterobacter cloacae, proteus mirabilis, bacteroides fragilis, staphylococcus epidermidis, propionibacterium acnes, candida albicans and/or malassezia furfur
A streptococcal, colonization technique for the prophylaxis and/or treatment of Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, Enterococcus faecium, Enterobacter cloacae, Proteus mirabilis, Bacteroides fragilis, Epidermal Staphylococcus, Propionibacterium acnes, Candida albicans, and/or Malassezia furfur-associated infection, colonization, or disease domain, capable of addressing pathogenic microbial colonization, commensal flora imbalance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
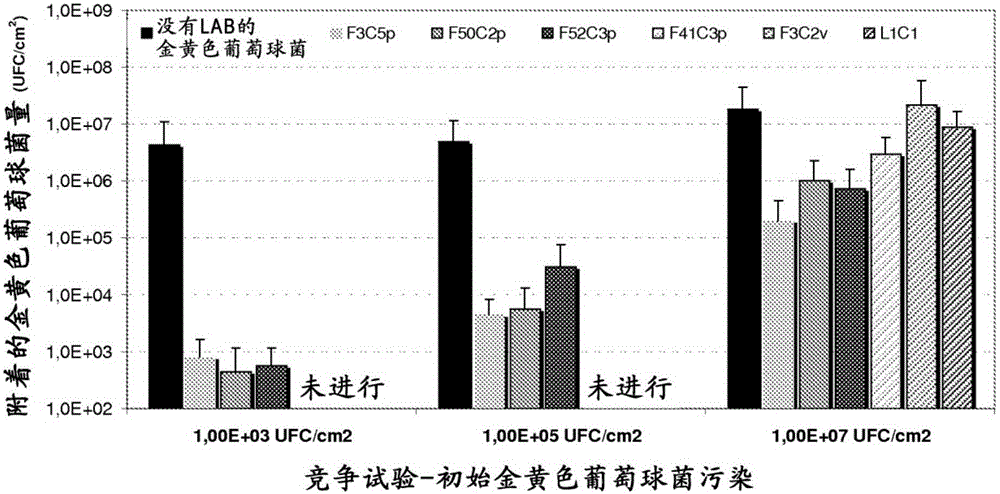
[0124] Example 1: Bacterial strain LactobacillussaniviriF3C5p (CNCMI-4650), Lactobacillus salivarius F50C2p (CNCMI-4651), Lactobacillus salivarius F52C3p (CNCM I-4652), Lactobacillus salivarius F41C3p (CNCMI-4653), Streptococcus light type F3C2v (CNCMI-4654) and Lactobacillus pentosus or Lactobacillus plantarum L1C1 (CNCMI-4655) Antibacterial activity
[0125] Lactobacillus saniviri F3C5p (CNCMI-4650), Lactobacillus salivarius F50C2p (CNCMI-4651), Lactobacillus salivarius F52C3p (CNCMI-4652), Lactobacillus salivarius F41C3p (CNCMI) were cultured in ManRogosaSharpe (MRS) medium at 37°C under anaerobic conditions -4653), Streptococcus light F3C2v (CNCMI-4654) and Lactobacillus pentosus or Lactobacillus plantarum L1C1 (CNCMI-4655) for 16 hours. Place 10 μl of these culture droplets on the surface of tryptone agar (TSA) medium. The tryptone agar (TSA) medium uses methicillin-resistant Staphylococcus aureus (MRSA) ATCC43300 or Pseudomonas aeruginosa ATCC9027 or Bacteroides fragil...
Embodiment 2
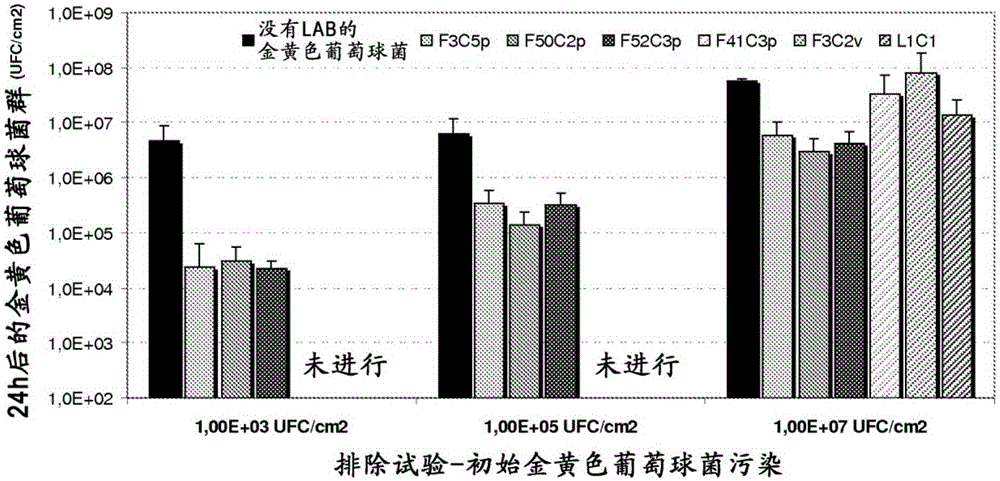
[0131] Example 2: Bacterial strain LactobacillussaniviriF3C5p (CNCMI-4650), Lactobacillus salivarius F50C2p (CNCMI-4651), Lactobacillus salivarius F52C3p (CNCM I-4652), Lactobacillus salivarius F41C3p (CNCMI-4653), Streptococcus light type F3C2v (CNCMI-4654) and Lactobacillus pentosus or Lactobacillus plantarum L1C1 (CNCMI-4655) Antibacterial activity after adding dressing
[0132] F3C5p, F50C2p, F52C3p, F41C3p, F3C2v and L1C1 bacteria were cultured in ManRogosaSharpe (MRS) medium at 37°C under anaerobic conditions for 16 hours. The dressing sheet (1cm×1cm) composed of polyurethane foam and lipid water glue net was used with 500μl of the culture solution of the bacteria according to the present invention (concentration of 10 9 -10 10 CFU.ml -1 ) Soaked and placed on the surface of tryptone agar (TSA) medium. The tryptone agar (TSA) medium is used for 10 6 -10 7 Two Staphylococcus aureus MRSAATCC43300 or Pseudomonas aeruginosa ATCC9027 cells were inoculated in advance in agar m...
Embodiment 3
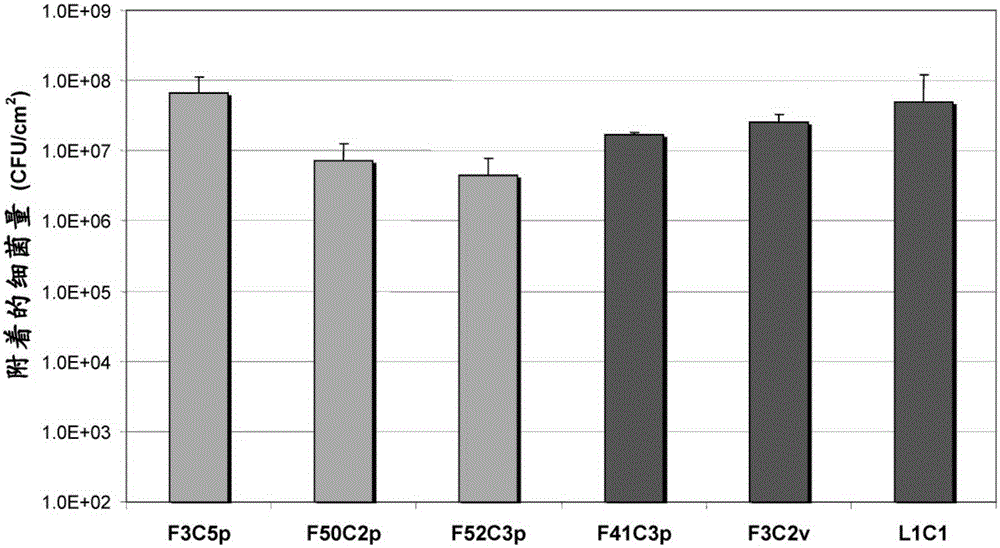
[0134] Example 3: Attachment ability of bacteria according to the present invention on collagen
[0135] EpiSkin (SkinEthic) type recombinant epidermis (1.07cm 2 Lactobacillus saniviri F3C5p, Lactobacillus salivarius F50C2p, Lactobacillus salivarius F52C3p, Lactobacillus salivarius F41C3p, Streptococcus light type F3C2v and Lactobacillus pentosus or Lactobacillus plantarum L1C1 bacteria were attached to the test. The insert containing the epidermis was placed in a 12-well plate containing 2 ml of maintenance medium, and incubated at 35°C±2°C for 24 hours to regenerate the epidermis.
[0136] After incubation, the maintenance medium was removed and 2ml of MRS medium was added. Then use the bacteria according to the invention (with a concentration of approximately 2.5x10 7 CFU.ml -1 ) Inoculate a medium that simulates wound exudate, called simulated wound fluid (50 / 50vol / vol) (described in Werthén et al., 2010). After incubating for 24 hours, wash with saline to remove unattached b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com