Application of lenalidomide to preparing medicine for relieving multiple sclerosis
A technology for multiple sclerosis and lenalidomide, which can be used in drug combinations, pharmaceutical formulations, allergic diseases, etc., and can solve problems such as lack of protection against multiple sclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
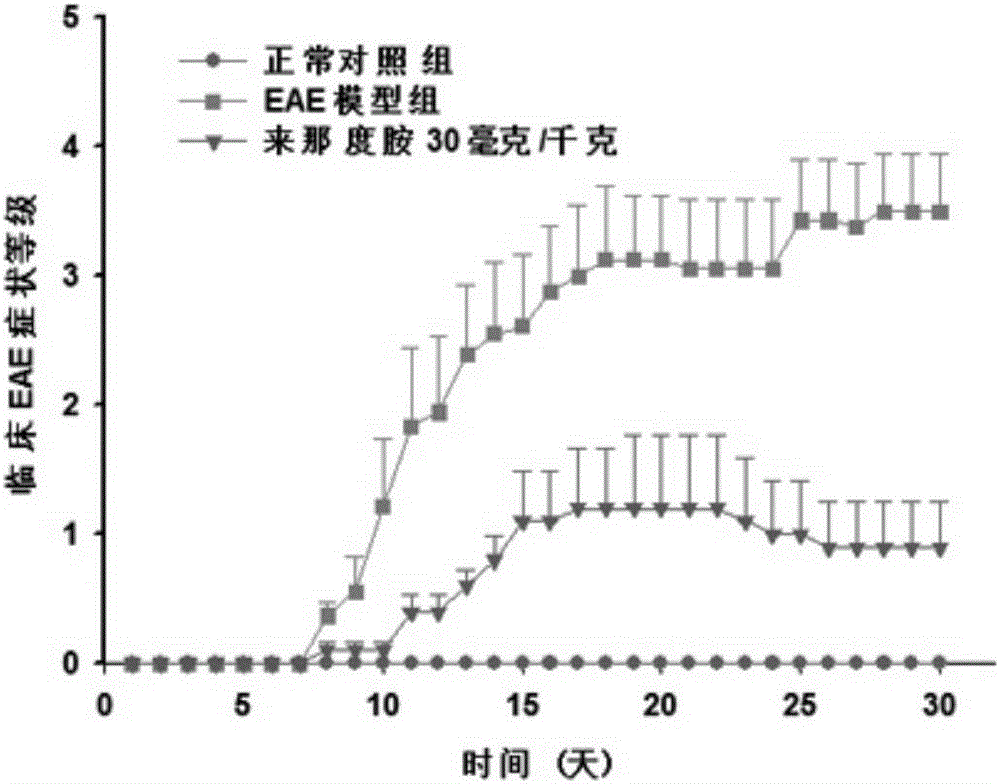
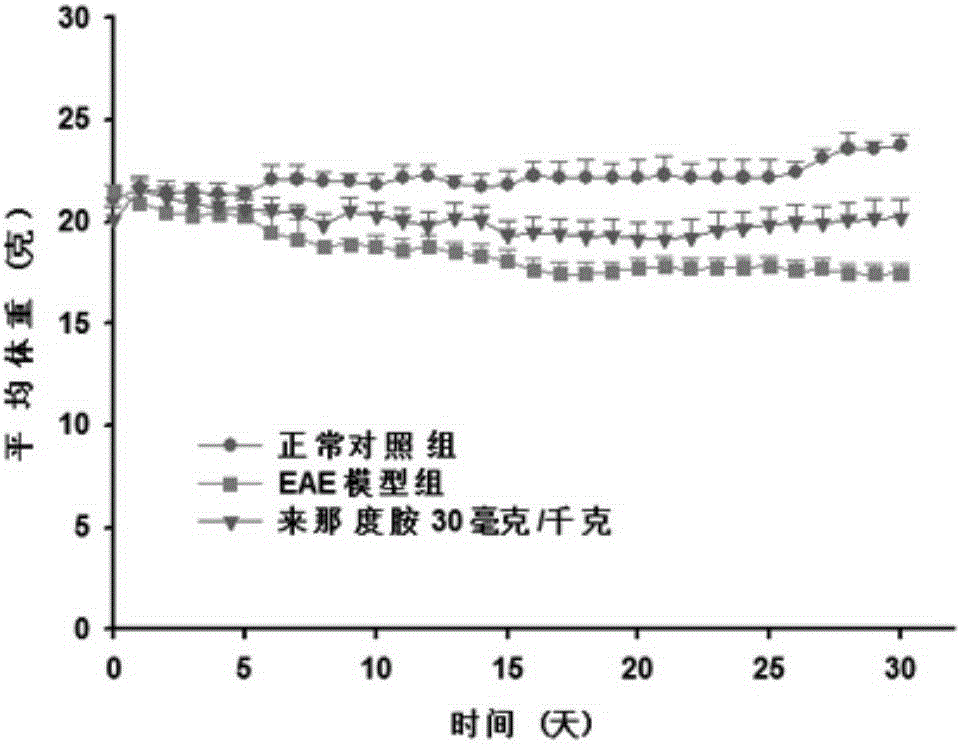
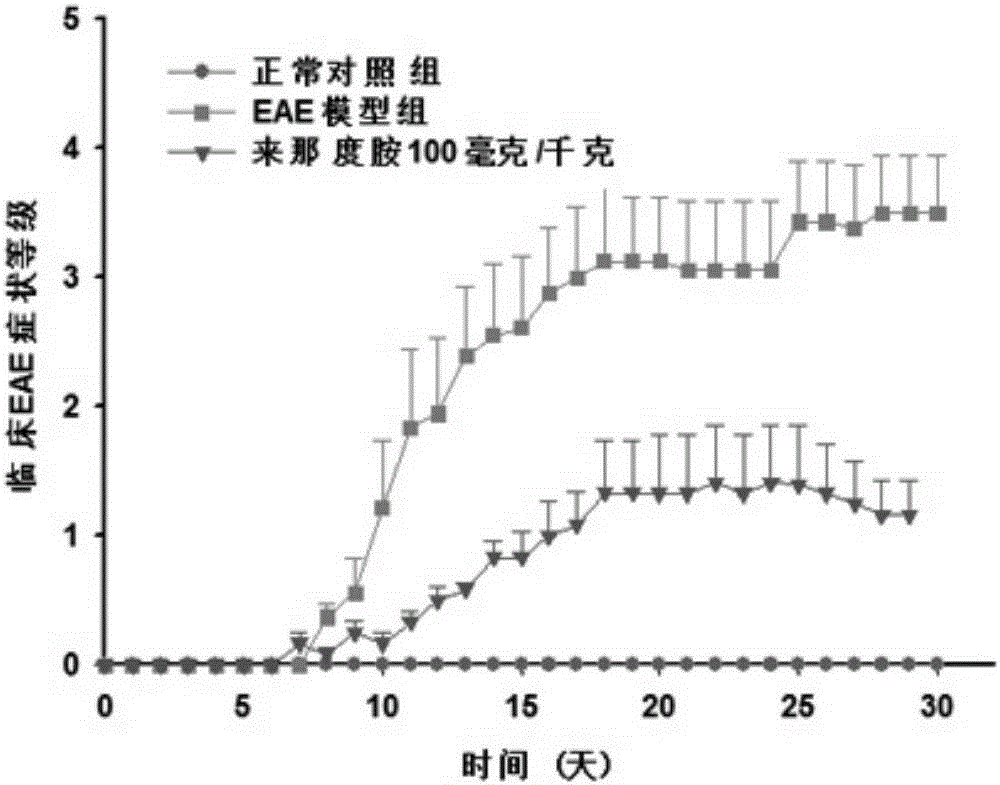
[0025] 50 C57BL / 6 adult female mice were used to make the EAE model. First, the mice were randomly divided into 6 groups, blank control group, EAE model group, EAE model group + lenalidomide low-dose administration group, EAE model group + lenalidomide There were 10 rats in each group of middle-dose lenalidomide administration group and EAE model group+lenalidomide high-dose administration group. Build EAE model: MOG first 35-55 Dissolve in sterile 1×PBS, the preparation concentration is 2mg / ml, dissolve Myco.Tuberculosis in complete Freund's adjuvant (CFA), the preparation concentration is 4mg / ml, vortex and mix. Then the two are mixed evenly by pumping and pushing back and forth through the syringe to prepare evenly dispersed immune antigens. Finally, 100 μl of the immune antigen was subcutaneously injected into the neck of the mouse, 50 μl of the immune antigen was injected subcutaneously into each of the left and right hind legs of the mouse, and 200 ng of pertussis toxin...
Embodiment 2
[0028] The spinal cords of each group on Day 23 were taken, frozen sections, and immunofluorescence. ⑴Take out the frozen section and dry it for 20 minutes; ⑵Wash 3 times with 1×PBS, 5 minutes each time; ⑶Blocking (1×PBS+5%NGS+0.3%Triton-100) for 1 hour; Preparation); (5) Wash 3 times with 1×PBS, 5 minutes each time; (6) Incubate with secondary antibody for 1 hour at room temperature in the dark (prepared with blocking buffer); (7) DAPI staining at room temperature for 5 minutes (in the dark); (8) Wash 3 times with 1×PBS, 5 minutes each time (protect from light); ⑼Seal the slide with antifade agent and store in the dark.
Embodiment 3
[0030] Paraffin-embedded the spinal cord and cross-sectioned the spinal cord with a thickness of 3 μm, and then stained with hematoxylin-eosin (Hematoxylin-Eosinstaining). ⑴ Dewax the paraffin sections with environmental transparent agent I for 15 minutes and environmental transparent agent II for 12 minutes; ⑷Stain in hematoxylin for about 2.5 minutes, rinse with tap water for about 15 minutes; ⑸Put the slices in 0.5% hydrochloric acid ethanol for color separation, see that the slices turn red, and the color is lighter; ⑹Wash for 5 minutes, ammonia water turns blue; ⑺Gradient dehydration , 70% ethanol for 2 minutes, 80% ethanol for 2 minutes, 90% ethanol for 2 minutes, 90% ethanol for 2 minutes; 95% ethanol for 2 minutes; Seal the slides after the agent is transparent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com