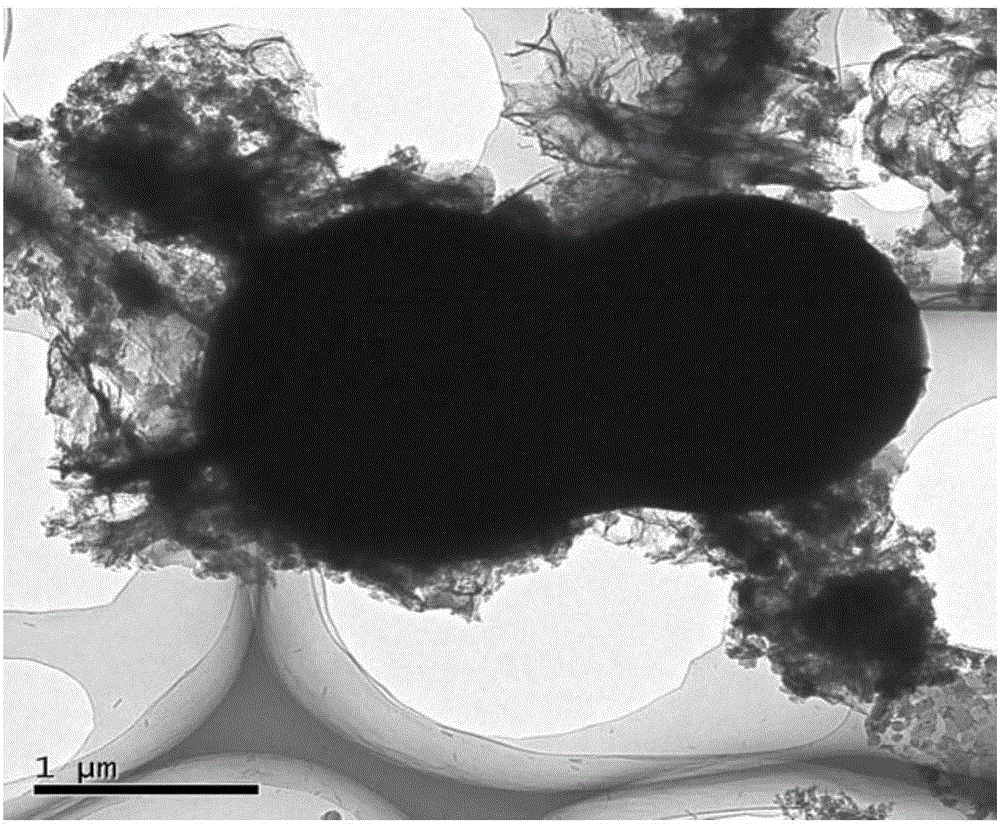
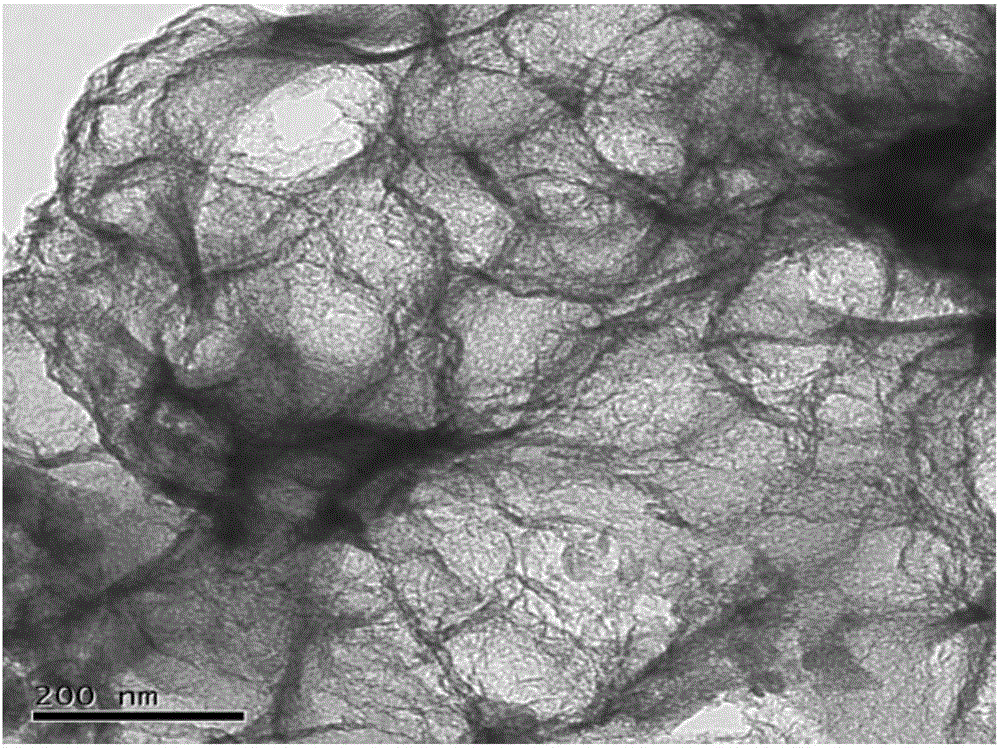
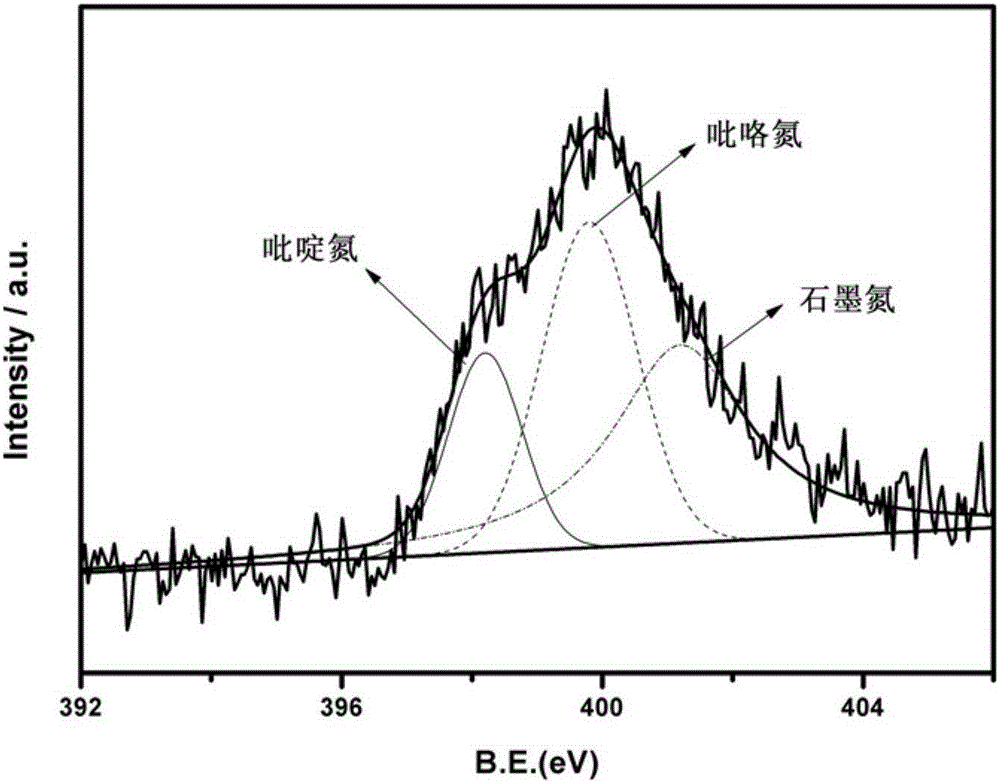
Nitrogen-sulfur co-doping carbon material with electro-catalysis oxygen reduction activity on acid and alkali conditions and preparation method
A nitrogen-sulfur co-doping, electrocatalytic oxygen technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of unsatisfactory oxygen reduction performance of fuel cell cathodes, and achieve the effect of high specific surface area and rich pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put 5g of clean chicken feathers into the lining of the reactor, pour 50g of analytically pure ammonia water (the mass ratio of chicken feathers to ammonia water is 1:10), and conduct a hydrothermal reaction at 150°C for 3h. After the hydrothermal kettle is cooled to room temperature, the ammonia solution in which the polypeptide is dissolved is heated in an electric furnace to remove ammonia, and then placed in an oven at 110°C until it is dried, and the obtained solid is ground with a mortar to obtain a solid powdery polypeptide. Weigh 2.5g of the above polypeptide powder into a beaker, add 60ml of methanol for ultrasonic dissolution (the mass ratio of polypeptide to methanol is 1:19), weigh 1.48g of zinc nitrate Zn(NO 3 ) 2 ·6H 2 O was poured into the above solution and stirred to obtain a suspension (that is, the mass ratio of the ligand to the complexing agent was 1:0.59), and the measured pH value of the suspension was about 4.15. The suspension was uniformly st...
Embodiment 2
[0040] Put 4g of clean chicken feathers into the lining of the reactor, pour 60g of analytically pure ammonia water (the mass ratio of chicken feathers to ammonia water is 1:15), and conduct a hydrothermal reaction at 150°C for 3h. After the hydrothermal kettle is cooled to room temperature, the ammonia solution in which the polypeptide is dissolved is heated in an electric furnace to remove ammonia, and then placed in an oven at 110°C until it is dried, and the obtained solid is ground with a mortar to obtain a solid powdery polypeptide. Weigh 2.5g of the above polypeptide powder into a beaker, add 55ml of methanol for ultrasonic dissolution (the mass ratio of polypeptide to methanol is 1:17.4), weigh 2.32g of zinc nitrate Zn(NO 3 ) 2 ·6H 2 O was poured into the above solution and stirred to obtain a suspension (that is, the mass ratio of the ligand to the complexing agent was 1:0.93), and 1M HNO was slowly added dropwise under the condition of magnetic stirring. 3 , and me...
Embodiment 3
[0043] Put 5g of clean chicken feathers into the lining of the reactor, pour 60g of analytically pure ammonia water (the mass ratio of chicken feathers to ammonia water is 1:12), and conduct a hydrothermal reaction at 140°C for 4h. After the hydrothermal kettle is cooled to room temperature, the ammonia solution in which the polypeptide is dissolved is heated in an electric furnace to remove ammonia, and then placed in an oven at 110°C until it is dried, and the obtained solid is ground with a mortar to obtain a solid powdery polypeptide. Weigh 2.5g of the above polypeptide powder in a beaker, add 50ml of methanol for ultrasonic dissolution (the mass ratio of polypeptide to methanol is 1:15.8), weigh 1.48g of zinc nitrate Zn (NO 3 ) 2 ·6H 2 O was poured into the above solution and stirred to obtain a suspension (that is, the mass ratio of the ligand to the complexing agent was 1:0.59), and 1M HNO was slowly added dropwise under the condition of magnetic stirring. 3 , and mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com