Preparation for Au-PtMnO2Co3O4CeO2 catalyst and catalytic application
A catalyst, catalytic oxidation technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve problems such as low conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] MnO with an atomic ratio of Co to Mn and Ce of 19 / 1 / 1 2 co 3 o 4 CeO 2 Preparation of composite oxides. Preparation of MnO with an atomic ratio of cobalt, manganese and cerium of 19 to 1 to 1 by precipitation oxidation 2 co 3 o 4 CeO 2 composite oxide catalyst. Under 333K and stirring conditions, 1mol / L Co(NO 3 ) 2 ·6H 2 O, Mn(NO 3 ) 2 ·6H 2 O and 1mol / L Ce(NO 3 ) 3 ·6H 2 O aqueous solution was slowly dropped into 0.2 mol / L sodium carbonate aqueous solution in proportion, the pH value was adjusted to 10.0 with 0.2 mol / L NaOH, and after aging at this temperature for 4 h, it was filtered and washed with deionized water. Then, the obtained precipitate was mixed again with deionized water and stirred, and an appropriate amount of H 2 o 2 (30%). After aging for 1h, filter and wash again with deionized water, dry at 383K for 12h, and bake at 538K for 4h.
Embodiment 2
[0016] Au-PtMnO 2 co 3 o 4 CeO 2 Catalyst preparation. Selected prepared MnO2Co 3 o 4 / CeO 2 Composite oxide is used as the carrier, gold is used as the active component, and the method of deposition and precipitation is adopted. According to gold, platinum loading MnO 2 co 3 o 4 CeO 2 The mass of composite oxides is 2% and 1% of the feed ratio to measure HAuCl 4 4H 2 O and H 2 PtCl 6 ·6H 2 O solution and adjusted to the appropriate concentration. With 0.1mol / L Na 2 CO 3 The solution was HAuCl 4 4H 2 O solution and Co 3 o 4 / CeO 2 Adjust the pH of the composite oxide suspension to an appropriate value, and then mix the HAuCl 4 4H 2 O and H 2 PtCl 6 ·6H 2 O solution was slowly dropped into MnO2Co 3 o 4 / CeO 2 complex oxide suspension. After aging at this temperature for 4 hours, it was filtered and washed with deionized water, dried at 353K for 24 hours, and then calcined at 443K for 4 hours under flowing air.
Embodiment 3
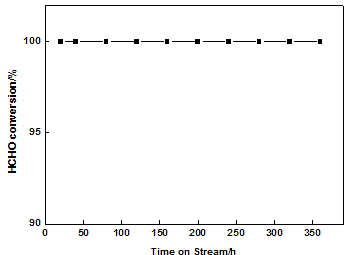
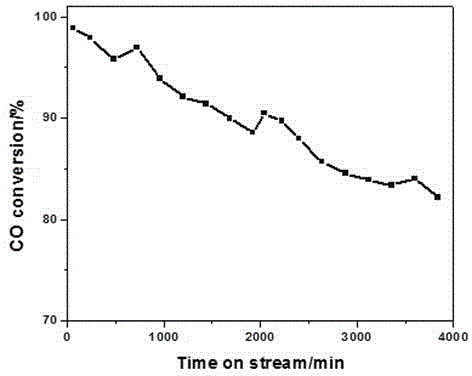
[0018] Au-PtMnO 2 co 3 o 4 CeO 2 Catalyst carbon monoxide and formaldehyde catalytic oxidation reaction performance test. The activity test of the catalyst was carried out in a miniature fixed-bed quartz tube reactor at atmospheric pressure. The catalyst powder is pressed into tablets, crushed and sieved to 40-60 mesh, and 200 mg is packed in a quartz reaction tube with Ф=6mm. The humidity condition is achieved by bubbling the reaction gas through a 298K constant temperature water bath (moisture content is 3.1vol%). Before the reaction, the catalyst was pretreated in air at 538K for 1 h. The gas at the outlet of the reactor was analyzed online with a HP-6890 gas chromatograph, and a nickel converter was installed in front of the FID detector, and CO and CO were separated in a hydrogen atmosphere. 2 Quantitatively converted to CH 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com