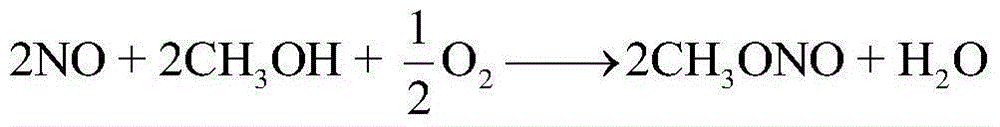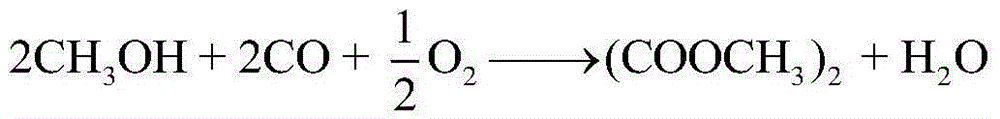Method for recovering methanol in coal-based synthetic gas-to-glycol process
A technology for the recovery of coal-based synthesis gas and methanol, which is applied in the preparation of hydroxyl compounds, chemical instruments and methods, and preparation of organic compounds, etc. The effect of reducing the consumption of methanol, reducing the amount of methanol circulation, and reducing the accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
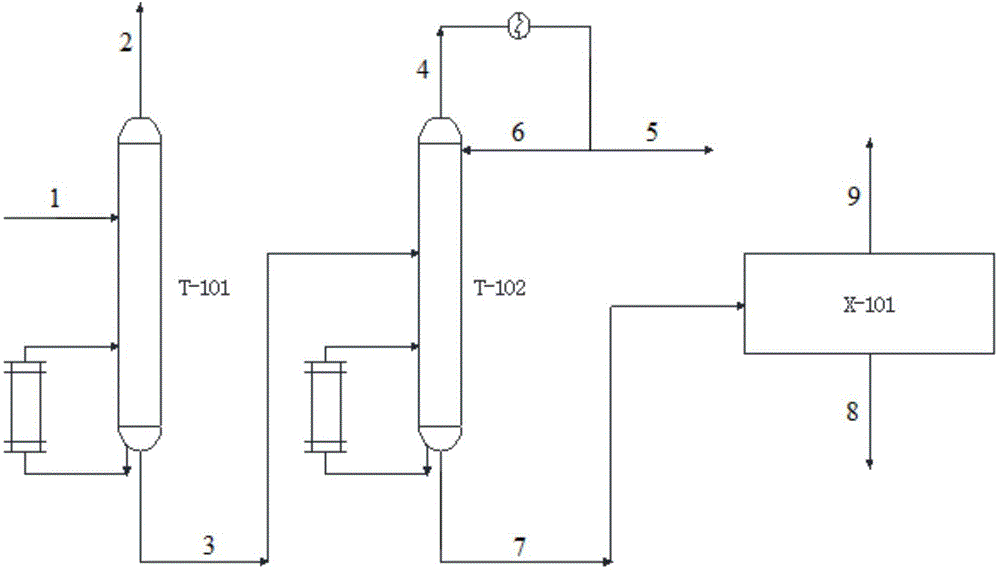
[0043] The crude methanol stream 1 containing methyl nitrite, methyl formate and dimethyl carbonate first enters the subester recovery tower T-101, and the top of the tower recovers to obtain methyl nitrite 2, and the methyl nitrite returns to the coupling reactor (marked in the figure), the still liquid 3 of the ester recovery tower T-101 enters the light removal tower T-102, and the top of the light removal tower T-102 removes methyl formate 5, and the still liquid of the light removal tower T-102 7 contains a large amount of methanol and a small amount of dimethyl carbonate, the mixture of methanol and dimethyl carbonate enters the methanol and dimethyl carbonate separation unit X-101, separates methanol 9 for recycling, and dimethyl carbonate 8 is extracted as a product.
[0044] 5000kg / hour of crude methanol, the content of methyl nitrite is 0.5wt%, the content of methyl formate is 0.5wt%, the content of dimethyl carbonate is 5wt%, and the content of methanol is 94wt%.
...
Embodiment 2
[0053] Embodiment is the same as [Example 1]. only,
[0054] 10000kg / hour of crude methanol, the content of methyl nitrite is 8wt%, the content of methyl formate is 20wt%, the content of dimethyl carbonate is 20wt%, and the content of methanol is 52wt%.
[0055] The theoretical plate number of the ester recovery tower is 25, the operating pressure at the top of the tower is 0.7MPag, the operating temperature at the top of the tower is 30°C, and the operating temperature at the bottom of the tower is 118°C.
[0056] The theoretical plate number of the light removal tower is 45, the operating pressure at the top of the tower is 0.7MPag, the operating temperature at the top of the tower is 100°C, and the operating temperature at the bottom of the tower is 129°C.
[0057] Among them, the main logistics components are shown in Table 2.
[0058] Table 2
[0059] logistics
1
2
3
5
7
9
8
temperature, ℃
40
30
118
40
12...
Embodiment 3
[0063] Embodiment is the same as [Example 1]. only,
[0064] 10000kg / hour of crude methanol, the content of methyl nitrite is 2wt%, the content of methyl formate is 1wt%, the content of dimethyl carbonate is 20wt%, and the content of methanol is 77wt%.
[0065] The theoretical plate number of the ester recovery tower is 15, the operating pressure at the top of the tower is 0.5MPag, the operating temperature at the top of the tower is 43°C, and the operating temperature at the bottom of the tower is 118°C.
[0066] The theoretical plate number of the light removal tower is 15, the operating pressure at the top of the tower is 0.3MPag, the operating temperature at the top of the tower is 75°C, and the operating temperature at the bottom of the tower is 105°C.
[0067] Among them, the main logistics components are shown in Table 3.
[0068] table 3
[0069] logistics
1
2
3
5
7
9
8
temperature, ℃
40
43
118
40
105 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com