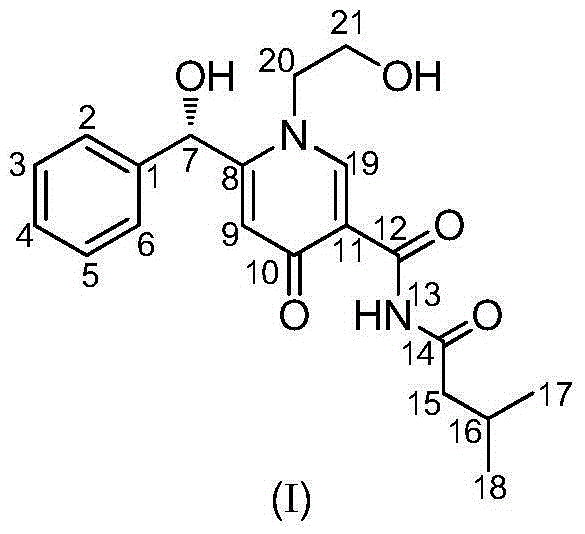
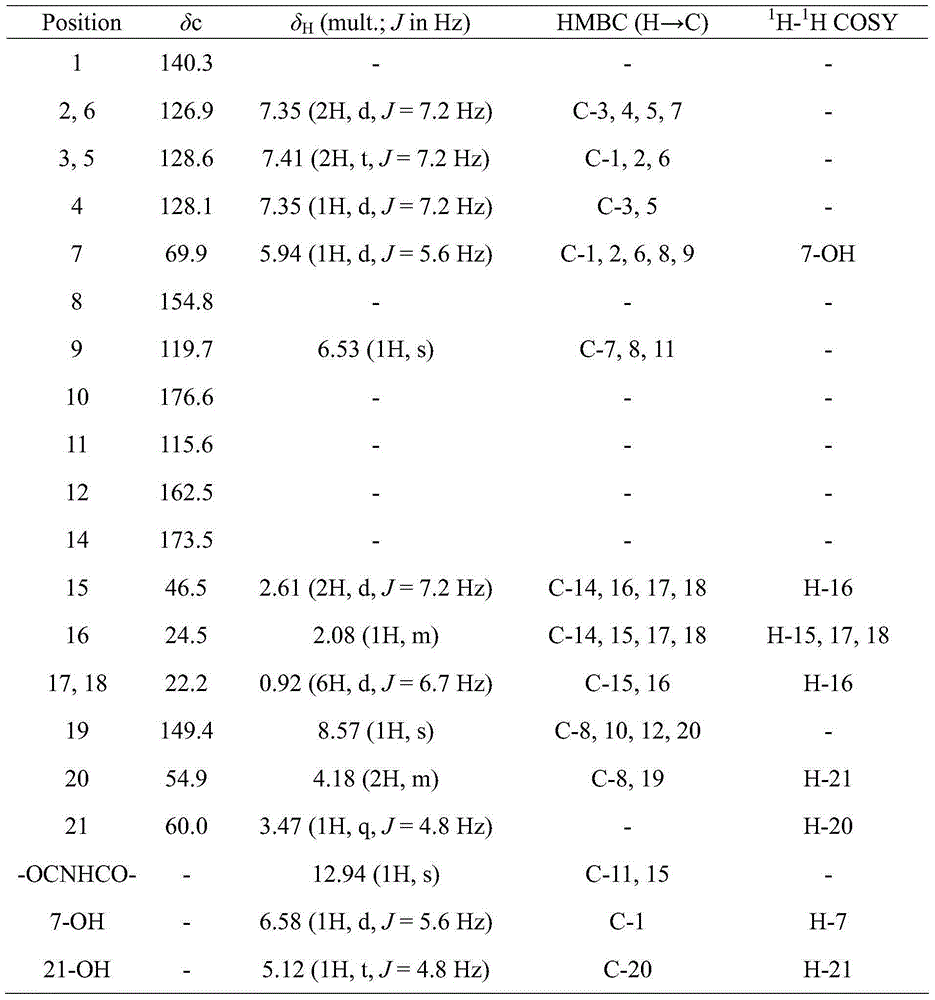
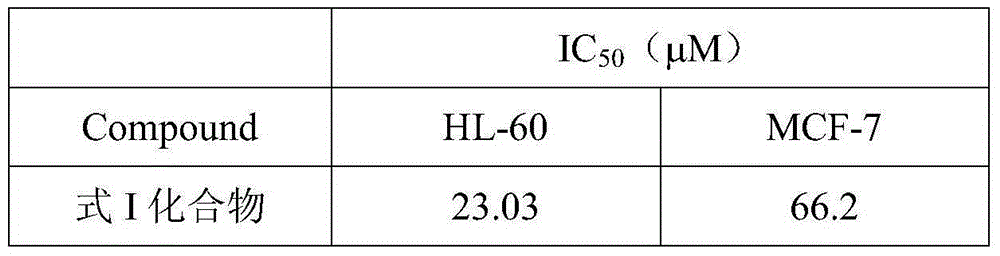
Novel pyridone alkaloid and preparation method therefor
An alkaloid and pyridone technology, applied in the field of medicine, can solve the problems that pyridone alkaloids have not been patented or reported in literature, and achieve the effects of inhibiting tumor cell growth, simple extraction and separation method, and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Fermentative production and separation and purification of the compound of formula I
[0020] 1 Fermentation production
[0021] Fermentation culture of production bacteria: After resuscitating the endophytic fungus Trametestrogii, transfer it from the inclined plane to 150mL culture medium [medium composition: mannitol 2%; glucose 2%; yeast extract 0.5%; peptone 1%; KH 2 PO 4 0.05%; MgSO 4 ·7H 2 0.03%; corn steep liquor 0.1%; tap water preparation] in the Erlenmeyer flask (500mL), at 28 ℃ shaker 180rpm vibration culture after 2 days as seed culture solution. Then the seed solution was inoculated into a 500mL Erlenmeyer flask containing 200mL of fresh, sterilized fungal No. 4 liquid medium according to the inoculum size of 5%, and was cultured statically at room temperature to obtain about 70L of fermentation broth.
[0022] 2 Obtaining of crude extract
[0023] The fermentation culture is filtered with 16 layers of gauze to separate the mycelium and ...
Embodiment 2
[0026] Embodiment 2: The growth inhibition test of the compound of formula I to human acute promyelocytic leukemia cell line HL-60 and human breast cancer cell MCF-7 in vitro:
[0027] HL-60 cells were cultured in RPMI1640 medium containing 10% heat-inactivated fetal bovine serum, 100 IU / mL penicillin, 100 mg / mL streptomycin, and 1 mmol / LL-glutamine at 37°C, 5% CO 2 Incubate in a saturated humidity incubator. Weigh trypan blue, add a small amount of distilled water to grind, add double distilled water to dilute to 4% storage concentration, filter with filter paper, and store at 4°C. For use, this stock solution was diluted to a working concentration of 0.4% with PBS. Take the above cells (1×10 5 / mL) was inoculated in a 12-well plate, 2 mL per well. Add different concentrations of drugs and incubate to prepare a single cell suspension. Take 50 μL of the cell suspension and add 50 μL of 0.4% trypan blue solution, mix well, and observe under a microscope within 3 minutes. Dea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com