Squarylium cyanine dye and preparation method therefor
A squaraine cyanine and dye technology, which is applied in the directions of organic dyes, chemical instruments and methods, methine-based/polymethine-based dyes, etc., can solve the problems of complicated synthesis, poor photostability of dyes, high price and the like, and achieves simple synthesis methods, The effect of good light stability and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
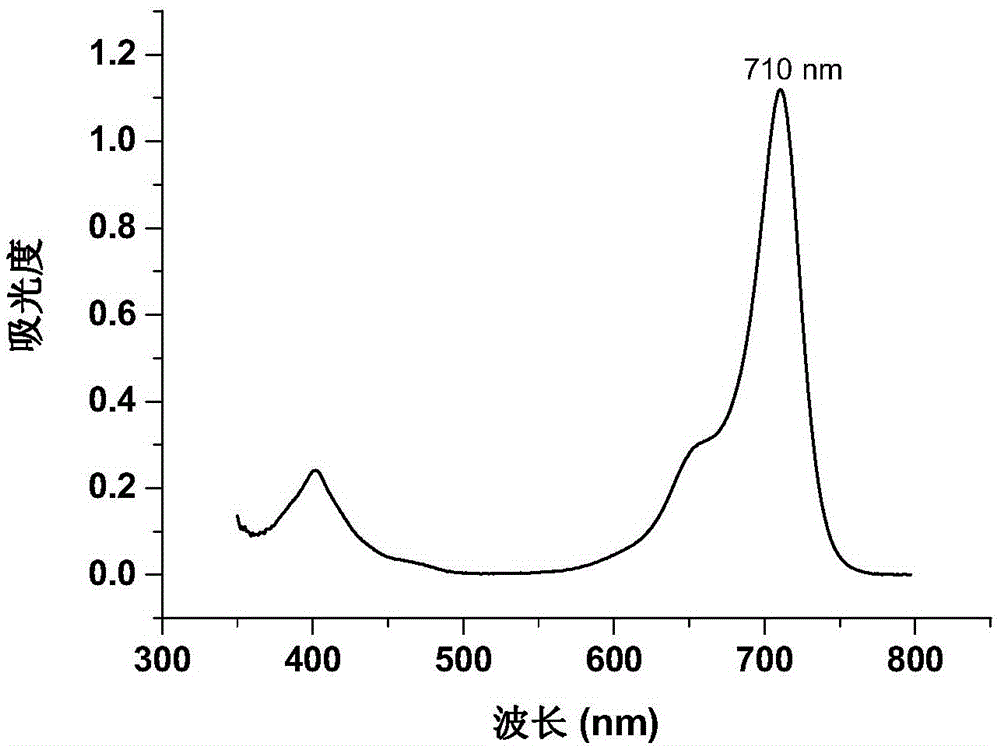
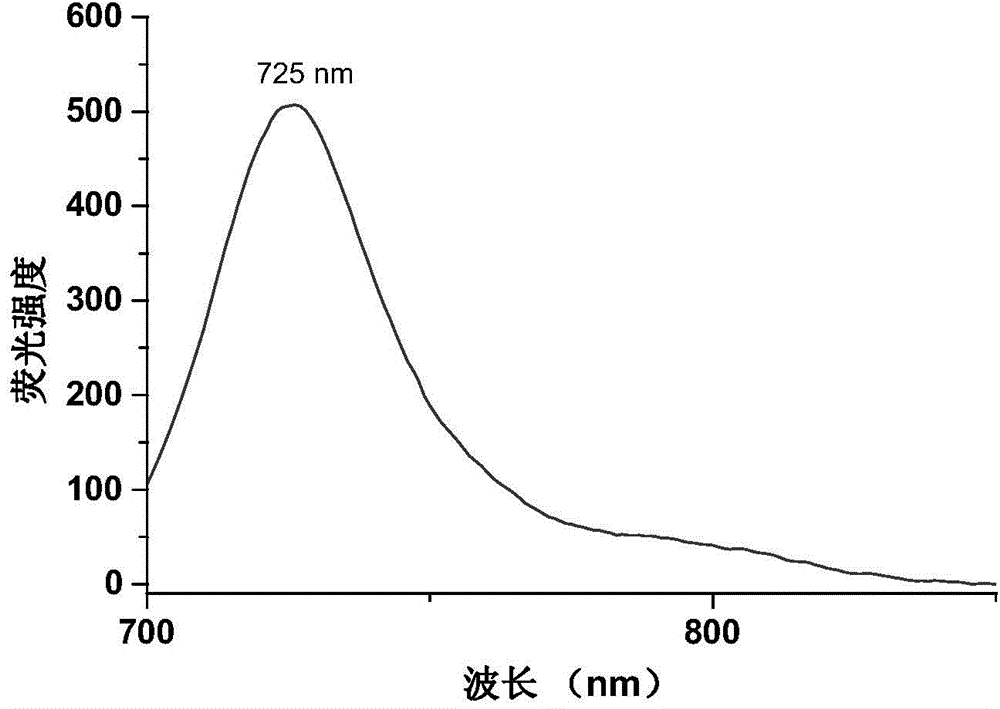
Image
Examples
Embodiment 1
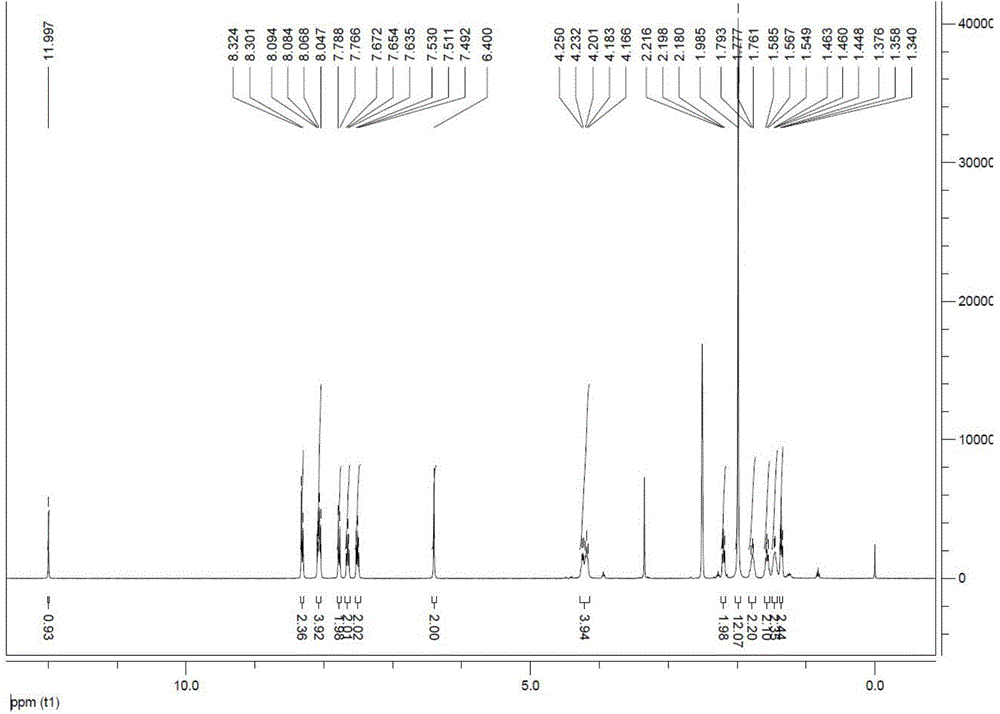
[0048] (1) Weigh 200mg of 2,3,3-trimethyl-4,5-benzindole and 490mg of n-bromohexanoic acid, add 15ml of nitromethane into a 50ml round bottom flask, vacuumize and protect with argon ; Stir, heat to reflux, overnight; spot the plate, the raw materials react completely. Cool to room temperature, add the reaction solution drop by drop into anhydrous ether, a dark green solid precipitates, filter with suction, add 50ml×3 ethyl acetate to the filter cake, heat and reflux to wash the drops, and the pure product [1] can be obtained. Yield 98%.
[0049] (2) Weigh 1g of 2,3,3-trimethyl-4,5-benzindole and 1.4ml iodoethane into a 50ml round bottom flask, add 15ml nitromethane, vacuumize and protect with argon ; Stir, heat to reflux, overnight; spot the plate, the raw materials react completely. Cool to room temperature, add the reaction solution drop by drop into anhydrous ether, a dark green solid precipitates, filter with suction, add 50ml×3 ethyl acetate to the filter cake, heat and...
Embodiment 2
[0056] (1) Weigh 200mg of 2,3,3-trimethyl-4,5-benzindole and 480mg of n-bromobutyric acid, add 15ml of toluene in a 50ml round bottom flask, vacuumize and protect with argon; stir , heated to reflux overnight; spot the plate, the raw materials reacted completely. Cool to room temperature, add the reaction solution drop by drop into anhydrous ether, a dark green solid precipitates, filter with suction, add 50ml×3 ethyl acetate to the filter cake, heat and reflux to wash the drops, and the pure product [1] can be obtained. Yield 92%.
[0057] (2) Weigh 1g of 2,3,3-trimethyl-4,5-benzindole and 1.5ml iodopropane, add 15ml toluene in a 50ml round bottom flask, vacuumize, protect with argon; stir, Heat to reflux overnight; spot the plate, the raw materials react completely. Cool to room temperature, add the reaction solution drop by drop into anhydrous ether, a dark green solid precipitates, filter with suction, add 50ml×3 ethyl acetate to the filter cake, heat and reflux to wash,...
Embodiment 3
[0062] (1) Weigh 200mg of 2,3,3-trimethyl-4,5-benzindole and 500mg of n-bromoheptanoic acid, add 15ml of nitromethane into a 50ml round bottom flask, vacuumize, and protect with argon ; Stir, heat to reflux, overnight; spot the plate, the raw materials react completely. Cool to room temperature, add the reaction solution drop by drop into anhydrous ether, a dark green solid precipitates, filter with suction, add 50ml×3 ethyl acetate to the filter cake, heat and reflux to wash the drops, and the pure product [1] can be obtained. Yield 95%.
[0063] (2) Weigh 1g of 2,3,3-trimethyl-4,5-benzindole and 1.5ml of iodobutane, add 15ml of nitromethane into a 50ml round bottom flask, vacuumize and protect with argon ; Stir, heat to reflux, overnight; spot the plate, the raw materials react completely. Cool to room temperature, add the reaction solution drop by drop into anhydrous ether, a dark green solid precipitates, filter with suction, add 50ml×3 ethyl acetate to the filter cake, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com