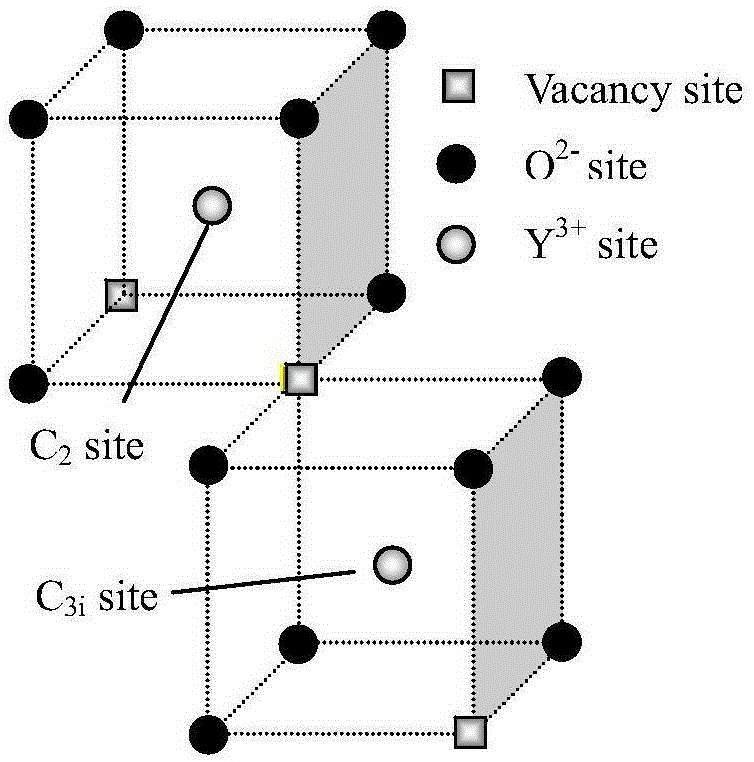
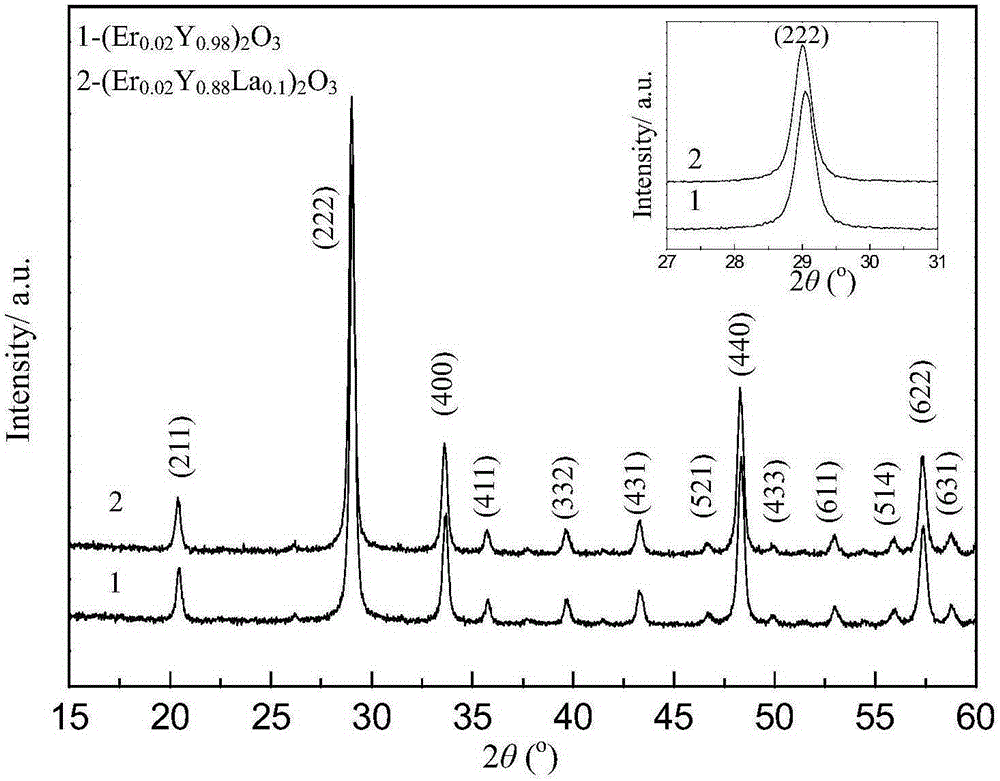
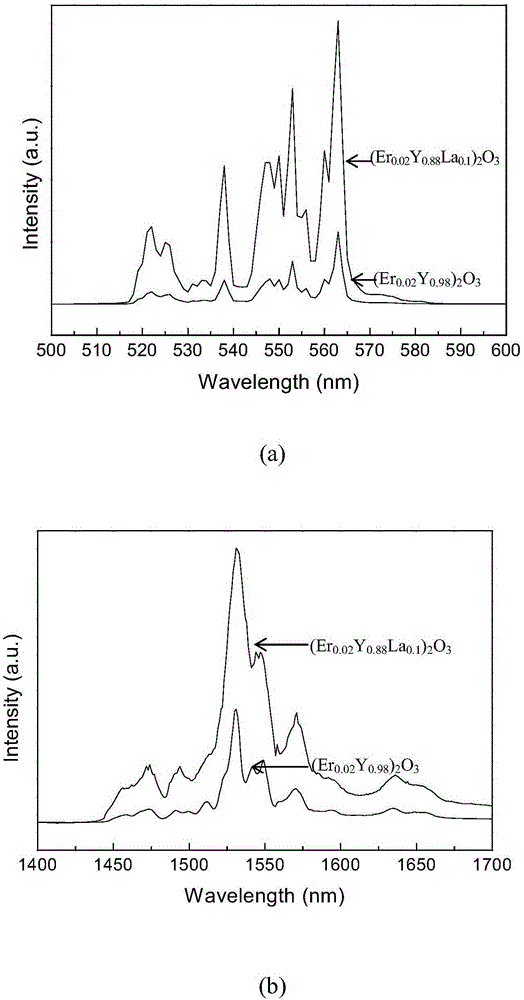
Erbium-doped yttrrium lanthanum oxide luminescent material and preparation method thereof
A luminescent material, the technology of lanthanum yttrium oxide, which is applied in the field of Er3+ doped luminescent material and its preparation, can solve problems such as difficult to balance, and achieve the effects of low production temperature, energy saving, and excellent luminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: (Er 0.02 Y 0.88 La 0.1 ) 2 O 3 preparation
[0032] Er(NO) with a concentration of 0.5 mol / L was used 3 ) 3 , Y(NO 3 ) 3 and La(NO 3 ) 3 The solution is the raw material, according to the chemical formula (Er 0.02 Y 0.88 La 0.1 ) 2 O 3 Accurately measure 4 ml of Er(NO 3 ) 3 solution, 176 ml Y(NO 3 ) 3 solution and 20 mL La(NO 3 ) 3 solution, mix and stir the above solution evenly; then add burning agent solid glycine, Er(NO 3 ) 3 , Y(NO 3 ) 3 and La(NO 3 ) 3 The ratio of the total molar amount of glycine to the molar amount of solid glycine is 1:2; stir on a magnetic stirrer at 80 °C to completely dissolve the solid glycine, forming a transparent mixed solution.
[0033] Then, the mixed solution was heated and dried in a constant temperature oven preheated to 120°C. When the solution turned into a transparent wet gel, the gel was put into a corundum crucible and transferred to a muffle furnace at 300°C. The gel appeared self-propaga...
Embodiment 2
[0039] Example 2: (Er 0.05 Y 0.87 La 0.08 ) 2 O 3 preparation
[0040] Er(NO) with a concentration of 0.5 mol / L was used 3 ) 3 , Y(NO 3 ) 3 and La(NO 3 ) 3 The solution is the raw material, according to the chemical formula (Er 0.05 Y 0.87 La 0.08 ) 2 O 3 Accurately measure 10 ml of Er(NO 3 ) 3 solution, 174 ml Y(NO 3 ) 3 solution and 16 mL La(NO 3 ) 3 solution, mix and stir the above solution evenly; add burning agent solid glycine, Er(NO 3 ) 3 , Y(NO 3 ) 3 and La(NO 3 ) 3 The ratio of the total molar amount of glycine to the molar amount of solid glycine was 1:4, and the solid glycine was completely dissolved by stirring on a magnetic stirrer at 70 °C to form a transparent solution.
[0041] Then, the mixed solution was heated and dried in a constant temperature oven preheated to 100°C. When the solution turned into a transparent wet gel, the gel was put into a corundum crucible and transferred to a muffle furnace at 400°C. The gel appeared self-pr...
Embodiment 3
[0046] Example 3: (Er 0.01 Y 0.89 La 0.1 ) 2 O 3 preparation
[0047] Er(NO) with a concentration of 0.5 mol / L was used 3 ) 3 , Y(NO 3 ) 3 and La(NO 3 ) 3 The solution is the raw material, according to the chemical formula (Er 0.01 Y 0.89 La 0.1 ) 2 O 3 Accurately measure 2 ml Er(NO 3 ) 3 solution, 178 ml Y(NO 3 ) 3 solution and 20 mL La(NO 3 ) 3 solution, mix and stir the above solution evenly; add burning agent solid citric acid, Er(NO 3 ) 3 , Y(NO 3 ) 3 and La(NO 3 ) 3 The ratio of the total molar amount of citric acid to the molar amount of solid citric acid is 1:2, and a transparent solution is formed after the solid citric acid is completely dissolved by stirring on a magnetic stirrer at 60 °C.
[0048] Then, the mixed solution was heated and dried in a constant temperature oven preheated to 80°C. When the solution turned into a transparent wet gel, the gel was put into a corundum crucible and transferred to a muffle furnace at 300°C. The gel a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com