Typing detection kit for human seasonal influenza viruses and application method thereof
A technology for seasonal influenza and detection kits, applied in biochemical equipment and methods, microbiological determination/inspection, etc., can solve the problems of poor PCR sensitivity, expensive dependence, unsuitable detection, etc., and achieve easy operation, low cost, High throughput effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
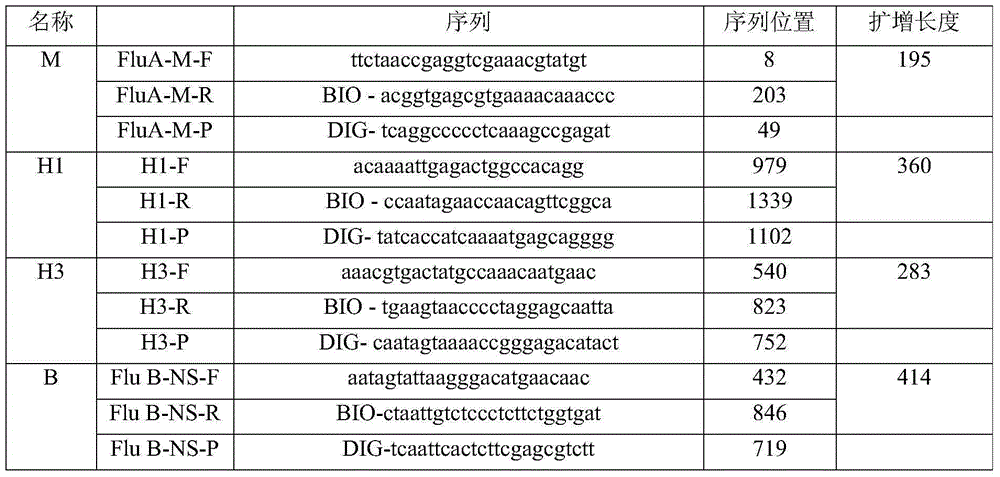
[0016] Embodiment 1: Target gene plasmid construction
[0017] Design specific primers for the M gene of influenza A virus, the HA gene of H1 subtype virus, the HA gene of H3 subtype virus and the NS gene of B virus respectively, and obtain four target gene fragments by PCR amplification. Connect with the T vector to obtain target gene positive plasmids, named as Pa-m, Ph1-ha, ph3-ha and pb-ns respectively.
Embodiment 2
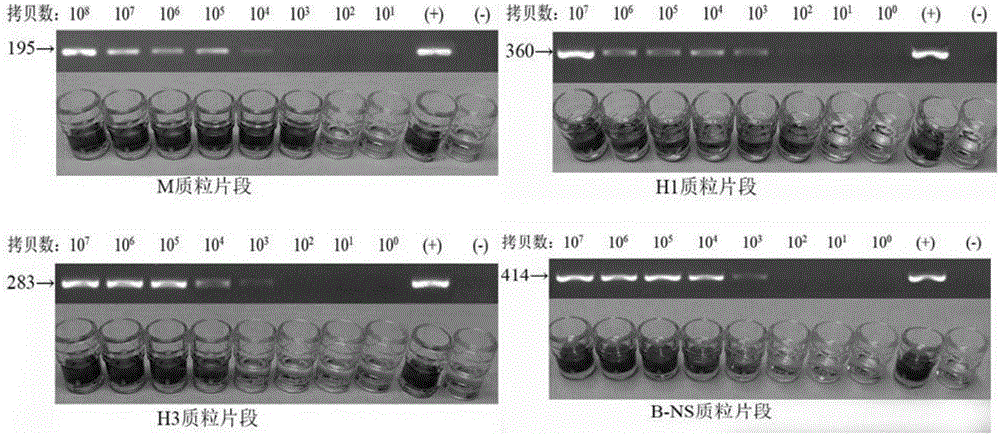
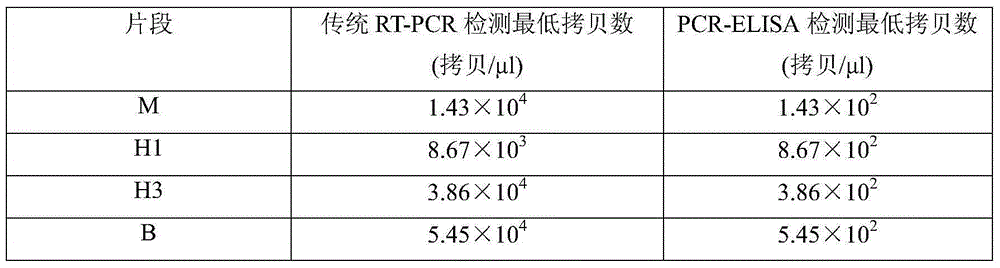
[0018] Embodiment 2: PCR-ELISA method sensitivity
[0019] (1) PCR reaction: Dilute the target gene cloning plasmids Pa-m, Ph1-ha, ph3-ha and pb-ns by 100 times, and then dilute to 10 times by 10 times. -2 -10 -9 A total of eight concentrations were used as templates, and the above biotin-labeled primers were used to perform PCR amplification reactions using the one-step kit from TaKaRa Company. The reaction conditions were: denaturation at 94°C for 30 s, annealing at 56°C for 30 s, extension at 72°C for 90 s, and after 35 cycles, extension at 72°C for 7 min to obtain biotin-labeled PCR products, and set a blank control group.
[0020] (2) ELISA detection: Take 5 μL of the amplified biotin-labeled PCR product, add it to 10 μL of 0.1mol / L NaOH solution, denature at room temperature for 10 minutes, and then add 10nmol / L digoxin-labeled specific probe (Dilute with hybridization solution containing 300mmol / L NaCl, 100mmol / LTris-HCl pH6.5, 10mmol / LEDTA, 0.1% Twee-20), incubate at...
Embodiment 3
[0024] Embodiment 3: PCR-ELISA method specificity
[0025] Using enterovirus, respiratory coronavirus, rhinovirus, respiratory syncytial virus, influenza A virus H1, H3, H5, H7 and H9 subtypes and influenza B nucleic acid as templates, PCR-ELISA experiments were performed with the above 4 sets of primers . Results The 4 sets of primers could not amplify the nucleic acid of enterovirus, respiratory coronavirus, rhinovirus, and respiratory syncytial virus, and there was no cross-reaction between different subtypes of influenza viruses, indicating that the established PCR-ELISA method had good specificity. sex. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com