Test method for hog cholera virus E2 protein quantification
A swine fever virus and detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effect of low requirements for personnel and equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1, the quantitative detection method of classical swine fever virus E2 protein, one, preparation and purification for the monoclonal antibody of classical swine fever virus E2 protein: 1. the hybridoma cell that will prepare monoclonal antibody is cultivated according to conventional culture mode, after culturing Healthy cells were centrifuged and adjusted to 1.0 x 10 6 / ml; ②The density-adjusted hybridoma cells were injected into the peritoneal cavity of Bacb / c mice, and the ascites in the peritoneal cavity of the mice was extracted 14 days later; ③After preliminary purification with saturated ammonium sulfate, further purification was performed with MelonGelIgGSpin Purification Kit; ④BCA reagent was used The box quantifies the purified monoclonal antibody, and it is ready for use after quantification.
[0013] 2. Optimization of coating, coating concentration, and concentration of CSFV E2 protein monoclonal antibody to be tested: ①Use the coating buffer to ...
Embodiment 2
[0015] Embodiment 2, the verification of specificity: get and express classical swine fever virus E2 protein, porcine circovirus (II) on insect cells, express porcine circovirus Cap protein on insect cells, porcine pseudorabies virus, porcine parvovirus, bovine The cell culture of viral diarrhea virus was tested, and the results showed that except for the positive expression of classical swine fever virus E2 protein on insect cells, the others were all negative, which proved that the coated monoclonal antibody had good specificity.
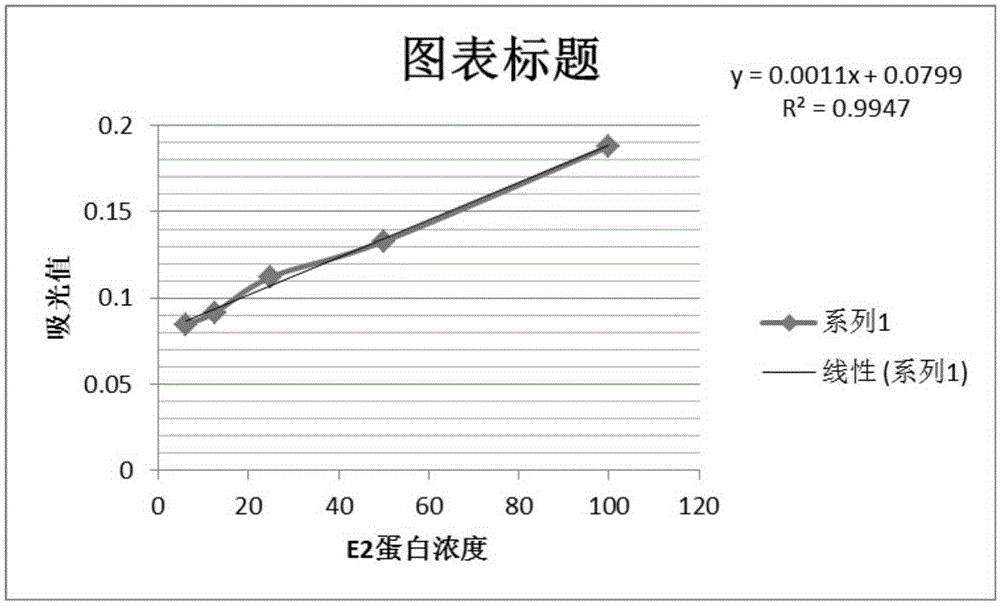
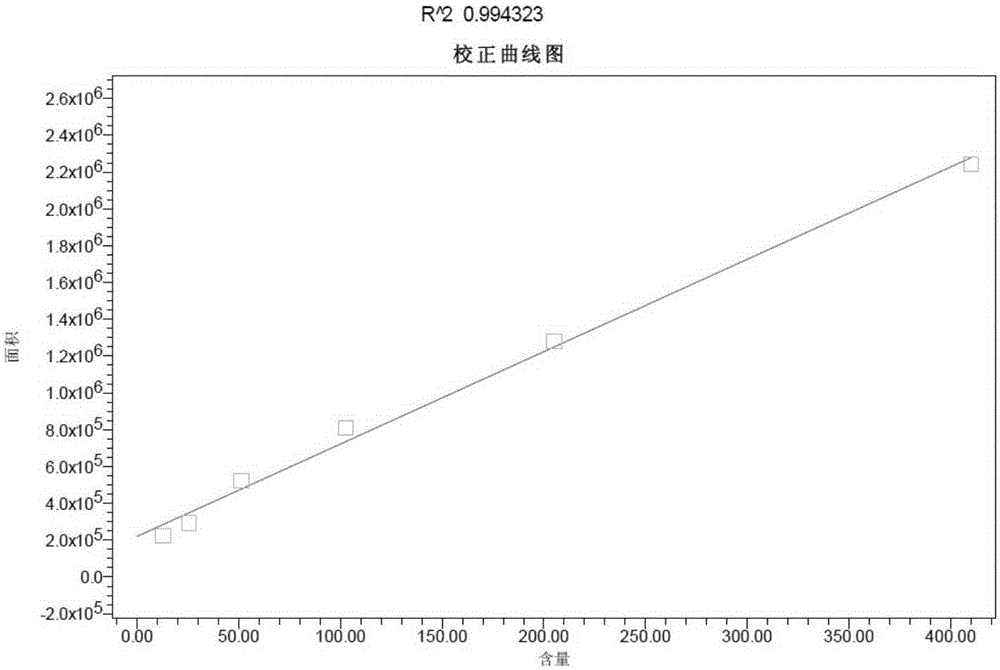
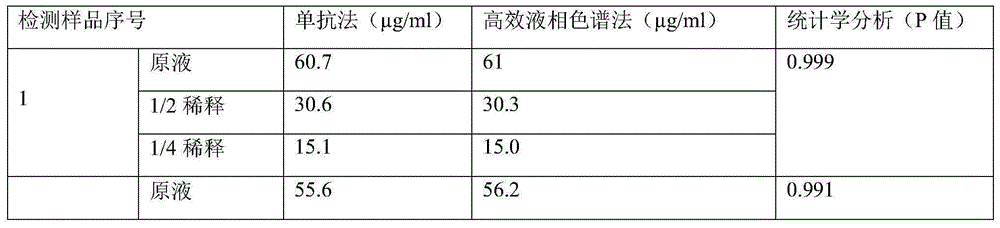
[0016] Quantitative accuracy verification of CSFV E2 protein: Randomly select 5 samples of CSFV E2 recombinant baculovirus expressed on insect cells for detection of CSFV E2 protein after dilution of stock solution, 1 / 2, and 1 / 4, respectively. The coated monoclonal antibody was detected by high-performance liquid chromatography, and the 1 / 2 and 1 / 4 diluted test results of the same sample were restored to the stock solution for statistical analysis....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com