Synthesis of diamine monomer with azo and polyimide prepared from diamine monomer
A technology of polyimide and diamine monomers, applied in the direction of azo dyes, diaryl/triarylmethane dyes, diaryl/triarylmethane dyes, etc., to achieve the effect of simple process operation and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
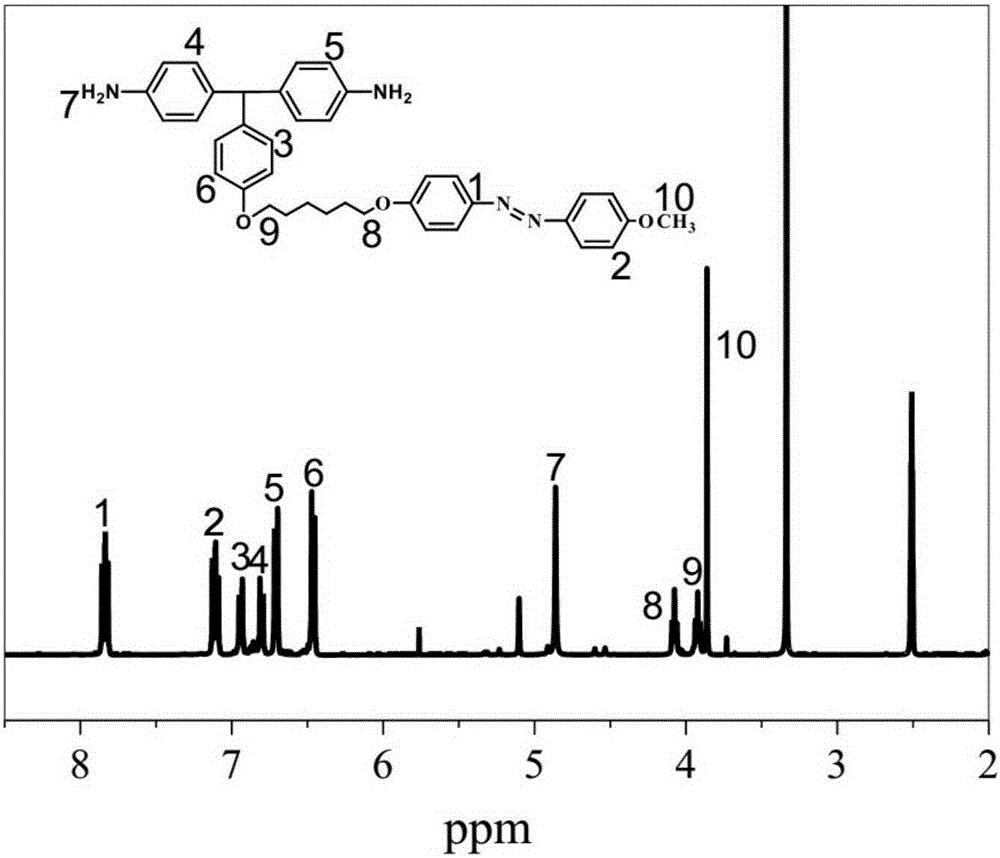
[0050] This example is to prepare 4,4`-diamino-4``azotriphenylmethane, that is, diamine monomer, whose structural formula is:
[0051]
[0052] This embodiment specifically includes the following steps:
[0053] Step 1, synthetic 4-hydroxyl-4 `-methoxy azobenzene, its structural formula is:
[0054] Specifically:
[0055] 1.1) 4-Methoxyaniline (6.1625g, 50mmol) was dissolved in 50mL concentrated HCl / H 2 O(V HCl :V H2O =1:4) in the beaker of the mixed solution, after it was completely dissolved, it was placed in an ice-water bath and cooled to below 5°C.
[0056] 1.2) Under vigorous stirring environment, 10mL of cold sodium nitrite (3.5237g, 50mmol) aqueous solution was added dropwise to this solution to obtain the diazonium salt solution of 4-methoxyaniline, and this solution was maintained at 5 below ℃.
[0057] 1.3) In another beaker, phenol (4.7012g, 50mmol) was dissolved in 25mL of 10% aqueous sodium hydroxide solution, cooled to below 5°C in an ice-water bath, ...
Embodiment 2
[0068] This embodiment is a polyimide prepared from the azodiamine-containing monomer described in Example 1, and its structural formula is:
[0069]
[0070] This embodiment specifically includes the following steps:
[0071] Step 1, feed nitrogen into the reactor equipped with mechanical stirring, nitrogen import and export and thermometer, the diamine monomer (3.0015g, 5mmol) of azo structure and diphenyl ether tetra-acid dianhydride (ODPA) single The polyamic acid solution (1.6263g, 5mmol) was dissolved in 10.8975g N,N-dimethylacetamide (DMAc), and reacted at 10°C for 8h to obtain a polyamic acid solution, the structural formula of which was:
[0072]
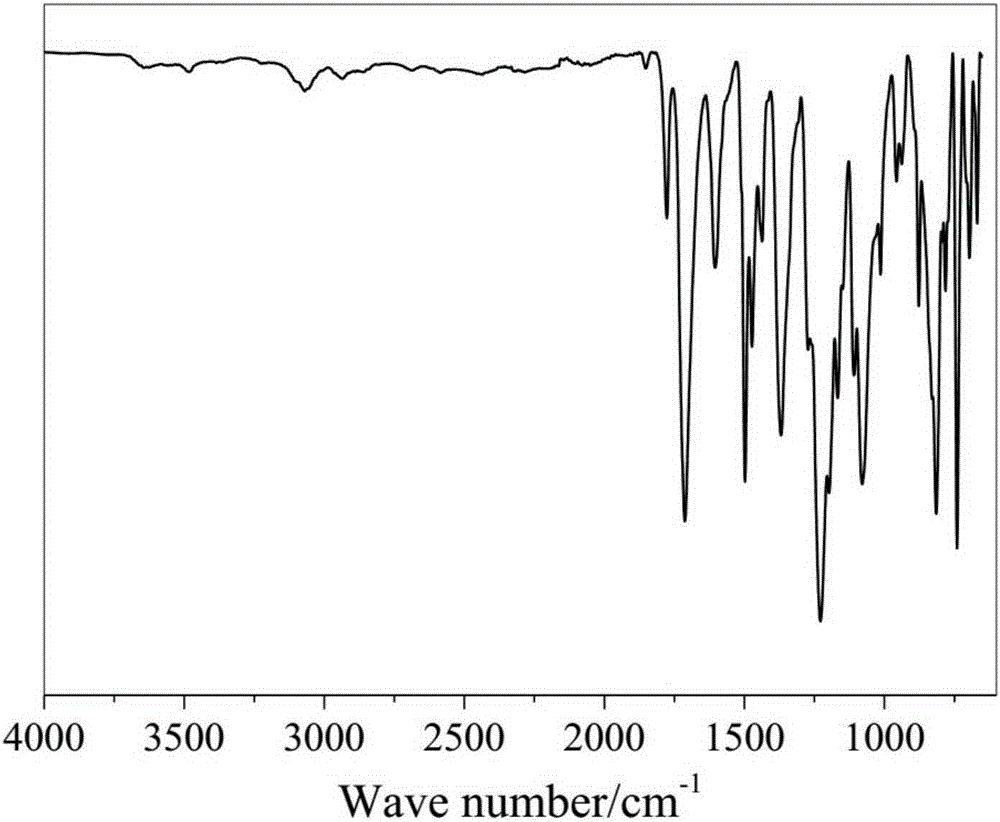
[0073] Step 2: Coating the polyamic acid solution at a speed of 3 cm / s, pre-baking at 50°C for 2 hours, then keeping the pre-baked film at 90°C for 2 hours, 120°C for 2 hours, and 200°C for 2 hours. 250 ° C for 2 hours, and finally obtained polyimide, the infrared spectrum of polyimide is as follows figure 2 shown....
Embodiment 3
[0075] This embodiment is a polyimide prepared from the azodiamine-containing monomer described in Example 1, and its structural formula is:
[0076]
[0077] This embodiment specifically includes the following steps:
[0078] Step 1, feed nitrogen into the reactor equipped with mechanical stirring, nitrogen inlet and outlet and thermometer, the diamine monomer (3.0056g, 5mmol) of azo structure and biphenyltetraacid dianhydride (BPDA) monomer ( 1.4705g, 5mmol) was dissolved in 10.9556g N,N-dimethylacetamide (DMAc), and reacted at 12°C for 10h to obtain a polyamic acid solution, whose structural formula is:
[0079]
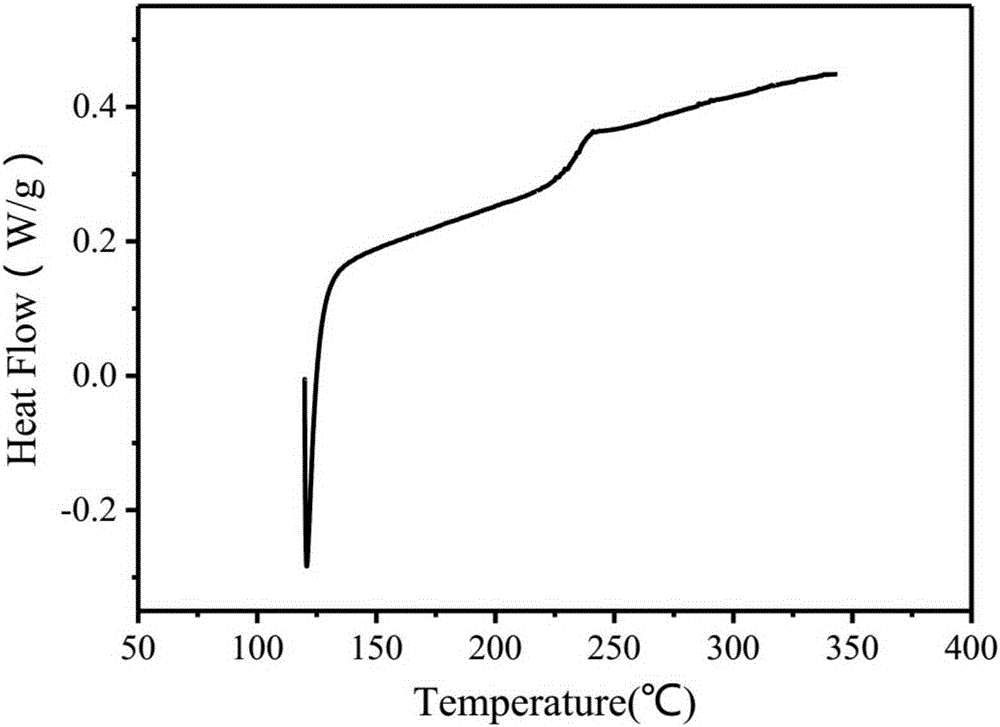
[0080] Step 2: Coating the polyamic acid solution at a speed of 3.5cm / s, pre-baking at 50°C for 2.5h, then keeping the pre-baked film at 90°C for 2.5h, 120°C for 2.5h, 200°C ℃ for 2.5 hours, 250 ℃ for 2.5 hours, and finally polyimide is obtained. The thermal weight loss curve of polyimide is as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com