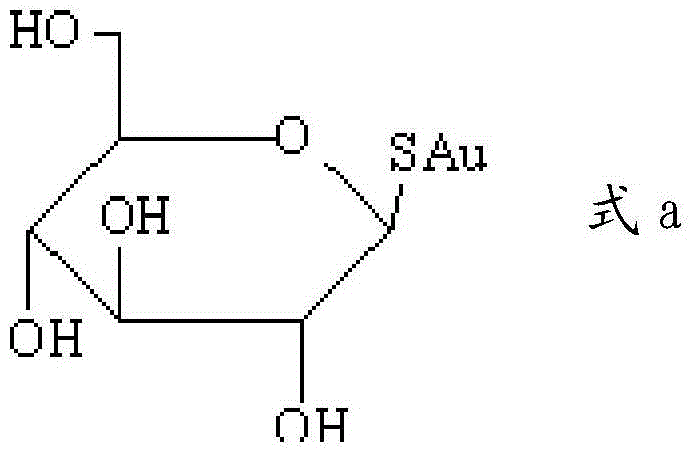
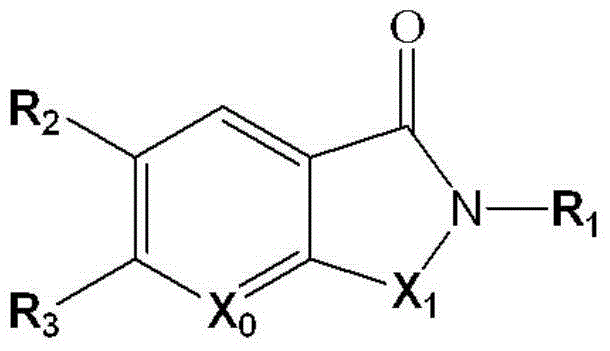
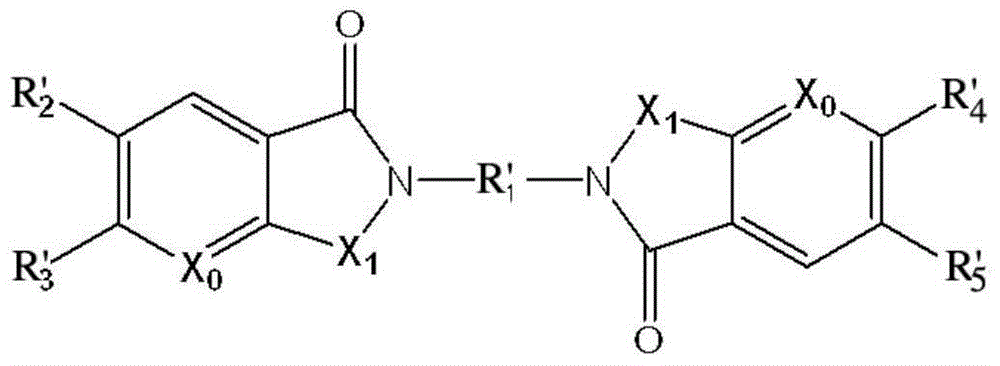
Application of detection reagent in preparation of drugs for evaluation of clinical tumor patient clinical treatment monitoring
A technology for tumor patients and clinical treatment, applied in the field of enzyme activity detection, can solve the problems of no ultra-early early warning monitoring of tumors, no public tumor treatment detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Determination of thioredoxin reductase activity in blood:
[0094] 1. Determination conditions of microplate reader
[0095] In the mode controlled by AscentSoftwareVersion2.6,
[0096] Oscillation: time 5s, speed 1020rpm;
[0097] Measurement mode: continuous;
[0098] Measurement Type: Dynamic;
[0099] Time interval: 30s;
[0100] Number of measurements: 15;
[0101] Wavelength: 405nm.
[0102] When analyzing an actual blood sample, a standard curve is established for each analysis run to calculate the concentration of the analyte in the analysis run.
[0103] 2. Measurement process
[0104] 2.1 Preparation of working solution: Mix 1MK2HPO4615ml and 1MKH2PO4385ml, adjust the pH to 7.0, add EDTA2924mg, BSA200mg, dissolve, and store at 4°C.
[0105] 2.2 Preparation of inhibitor working solution (25 μl): Dilute 20 mM compound I stock solution to 25 μM with working solution, and store at 4° C. in the dark.
[0106] 2.3 Turn on the microplate reader to preheat in...
Embodiment 2
[0116] Kits for the determination of thioredoxin reductase activity in blood:
[0117] Test kit of the present invention may contain:
[0118] 1. DTNB powder 49.8mg, protected from light, stored at room temperature;
[0119] 2. NADPH powder 4.2mg, protected from light, stored at -20°C;
[0120] 3. 10×working solution (1M potassium phosphate buffer, pH=7.4, containing 73.1mgEDTA, 5mgBSA.) 2mL, store at 4°C;
[0121] 4. TrxR inhibitor solution (containing 2.5mM inhibitor selected from one of the compounds I-XI), 100uL, stored at -20°C;
[0122] 5. Positive control: rat liver TrxR, 300U / mgXXUnit, stored at -20°C;
[0123] 6. TNB standard;
[0124] The detection reagent can be used for 100 tests;
[0125] The using method of kit of the present invention:
[0126] 1. Take 2mL of 10× working solution (0.5M potassium phosphate buffer, pH7.4) and dilute to 20mL with deionized water (containing 73.1mg ethylenediaminetetraacetic acid (EDTA), 5mg bovine serum albumin (BSA) )) to o...
Embodiment 3
[0141] 1. Detection data of TR activity in normal group and tumor group
[0142] Table 2 Statistics of TR activity detection data in normal group and tumor group
[0143]
[0144]
[0145] 2. Comparison test between normal group and tumor group (comparison test of two normal samples) F test
[0146] TR (正常) ~N(μ TR(正常) , σ 2 TR(正常) )
[0147] TR (肿瘤) ~N(μ TR(肿瘤) , σ 2 TR(肿瘤) )
[0148] F=(S 2 TR(肿瘤) / σ 2 TR(肿瘤) ) / (S 2 TR(正常) / σ 2 TR(正常) )≈S 2 TR(肿瘤) / S 2 TR(正常) ~F(n (肿瘤) -1,n (正常) -1)
[0149] Calculate F=S 2 TR(肿瘤) / S 2 TR(正常)
[0150] Judgment F﹤F α / 2 (n (肿瘤) -1,n (正常) -1), P>α, no significant difference between the two
[0151] Judgment F≥F α / 2 (n (肿瘤) -1,n (正常) -1), P<α, there is a significant difference between the two
[0152] Table 3 Comparison test between normal group and tumor group
[0153]
[0154] 3. Comparison test between normal group and tumor group (comparison test of two normal samples) u test
[0155] Means com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com