Synthetic method and micrometer wire preparation method of perylene bisimide derivative
A technology of perylene imide and synthesis method, which is applied in the fields of synthesis of perylene imide derivatives and preparation of micron wires, can solve problems such as no reports, and achieve the effects of high product purity, few by-products and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The present invention is further illustrated by the following examples.
[0023] The synthetic method of described perylene imide derivative:
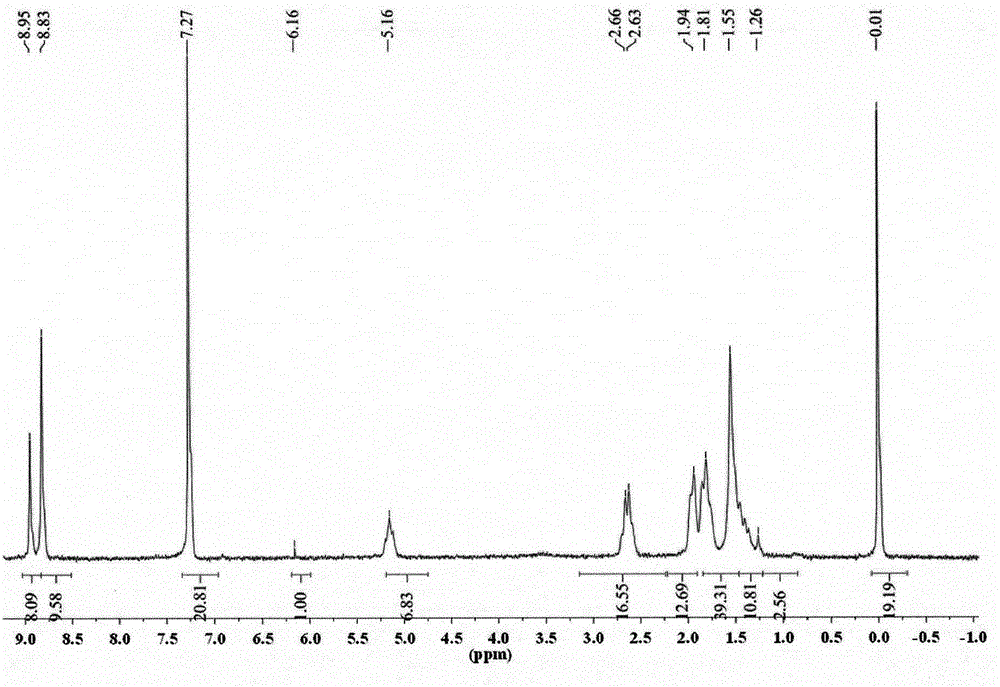
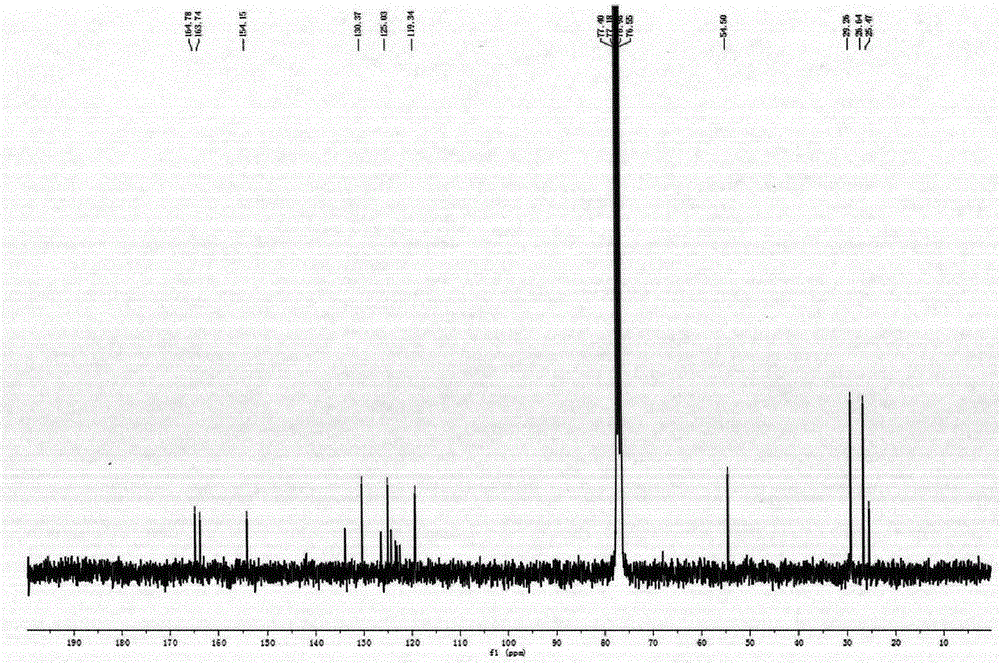
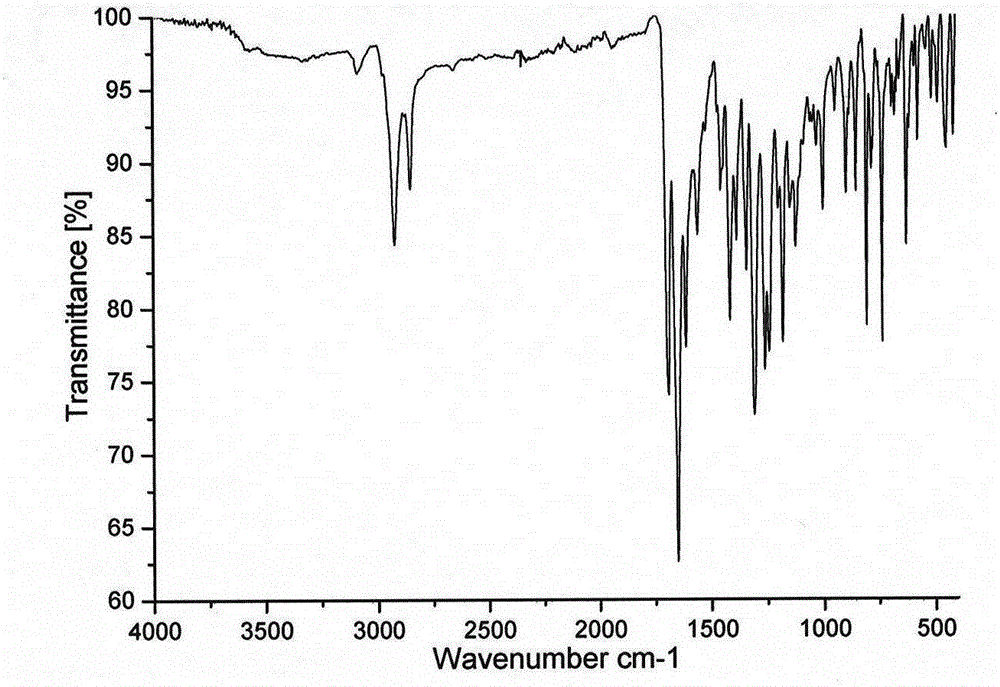
[0024] 1-NO 2 -3,4; 9,10-perylenetetracarboxylic acid diimide (0.6g, 1mmol) was dissolved in 60mL of N-methylpyrrolidone, under vigorous stirring, heated at 180°C for 5 hours, and the reacted solution After cooling, drop it into 500mL hydrochloric acid solution with a concentration of 2moL / L to precipitate a precipitate, vacuum filter the precipitate, wash the solid with distilled water, and dry it in vacuum. phase, purified by silica gel column chromatography to obtain 0.34 g of a bright yellow solid product with a yield of 60%. 1 H-NMR (CHCl 3 , TMS, ppm): δ=8.95 (d, 2H), 8.83 (d, 4H), 5.16 (m, 2H), 2.66-2.63 (m, 4H), 1.94-1.55 (m, 8H), 1.41-1.26 (m, 8H). 13 CNMR (75MHz, CDCl 3 , ppm): δ=164.78, 163.74, 154.15, 133.93, 130.38, 126.46, 125.03, 124.36, 119.34, 54.50, 29.27, 26.65, 25.48.FT-IR (KBr, cm -1 ): v=2922, 1689, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com