Preparation method for ardisia mamillata triterpenoid saponin reference substance
A technology of abatin and pyranoside, which is applied in the field of medicine, can solve problems such as unseen preparation methods, and achieve the effect of non-toxic, non-polluting solvents and less organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of reference substance
[0024] 1) Take Hutonghong medicinal material, crush it through a 10-mesh sieve, add 10 times the amount of 80% ethanol to reflux for extraction for 2 hours, repeat the extraction 3 times, filter, combine the filtrates and recover the ethanol under reduced pressure to obtain Hutonghong extract;
[0025] 2) Put the extract on a D141 macroporous adsorption resin column, wash with water until the eluent is nearly colorless, discard the eluent; elute with 30% ethanol until the eluent is nearly colorless, discard the eluent; use 80 % ethanol to elute until the eluate is nearly colorless, collect the eluate, concentrate and dry under reduced pressure to obtain tiger tongue red extract;
[0026] 3) Mix the extract from 2) with an equal amount of silica gel, dry and finely grind it and pour it evenly into the upper end of the packed silica gel column, slowly add the mobile phase ethyl acetate:methanol (7:3) at an appropriate flow rate Car...
Embodiment 2
[0045] 1 Influence of the extraction process of tibatin B on the extraction rate
[0046] 1) The influence of the extraction method on the extraction rate
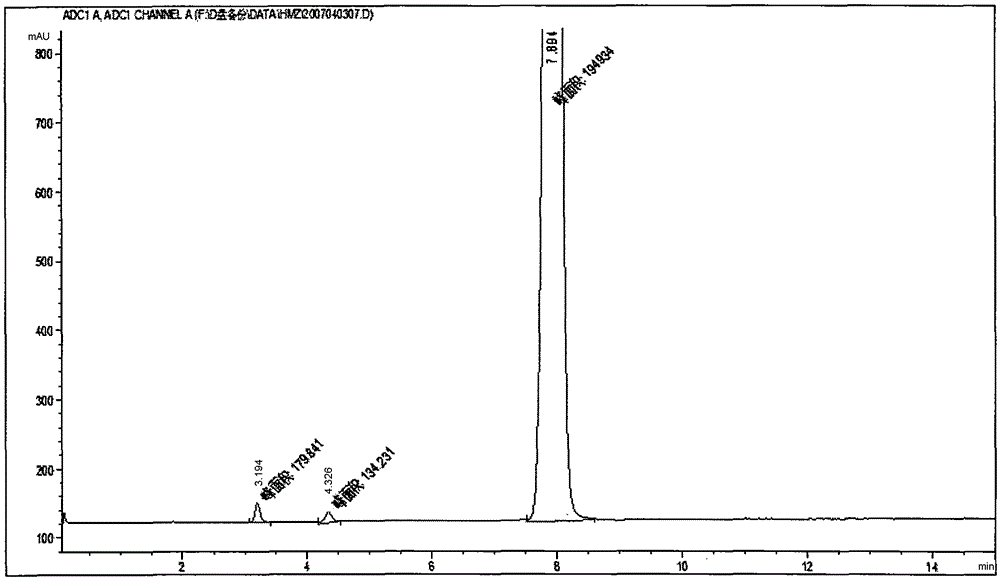
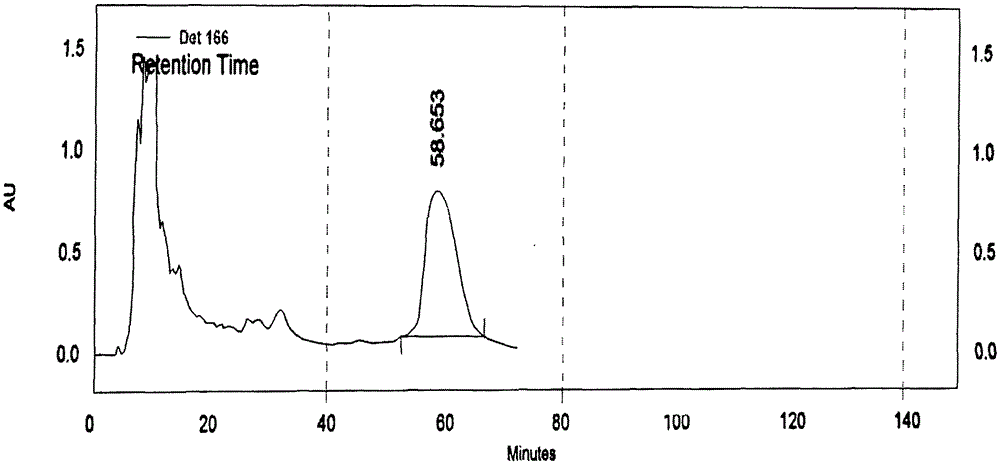
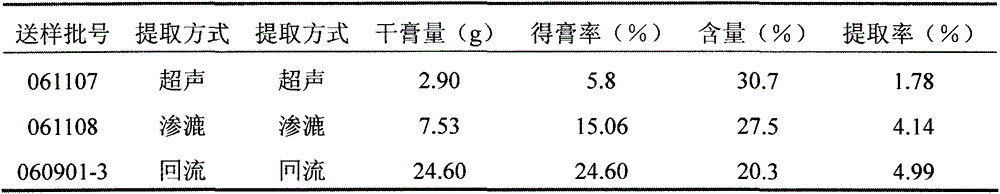
[0047] Take 3 parts of 10-mesh tiger tongue red medicine powder, each 50.00g. Add 10 times the amount of 80% ethanol respectively, after soaking for 30 minutes, one part was ultrasonically extracted at room temperature for 30 minutes; one part was reflux extracted for 30 minutes; The extract was filtered through a 200-mesh sieve, and the filtrate was concentrated under reduced pressure. The concentrate was evaporated to dryness in a water bath. The dry paste was dried in an oven at 70°C under reduced pressure for 8 hours, and the content of bechatin B was determined. The results are shown in Table 1.
[0048] Table 1 Comparison of extraction methods
[0049]
[0050] 2) The influence of extraction solvent on the extraction rate of tiger lipin B
[0051] Take 5 parts of tiger tongue red powder of 10 mesh, 50.00 g ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com