Specifically marked capsaicin fluorescent probe and synthetic method and application thereof
A technology of fluorescent probe and synthesis method, which is applied in the field of capsaicin fluorescent probe and its synthesis, can solve the problems of inability to visualize the dynamic research of TRPV1 channel, lack of fluorescence characteristics, etc., and achieves long excitation wavelength, mild labeling, and good fat solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
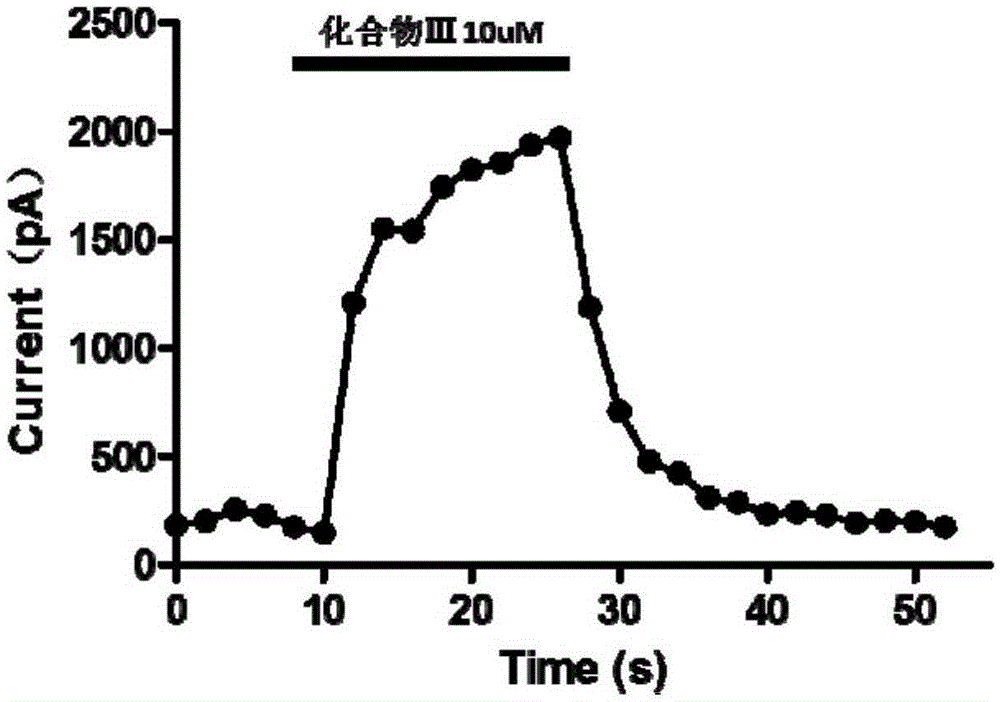
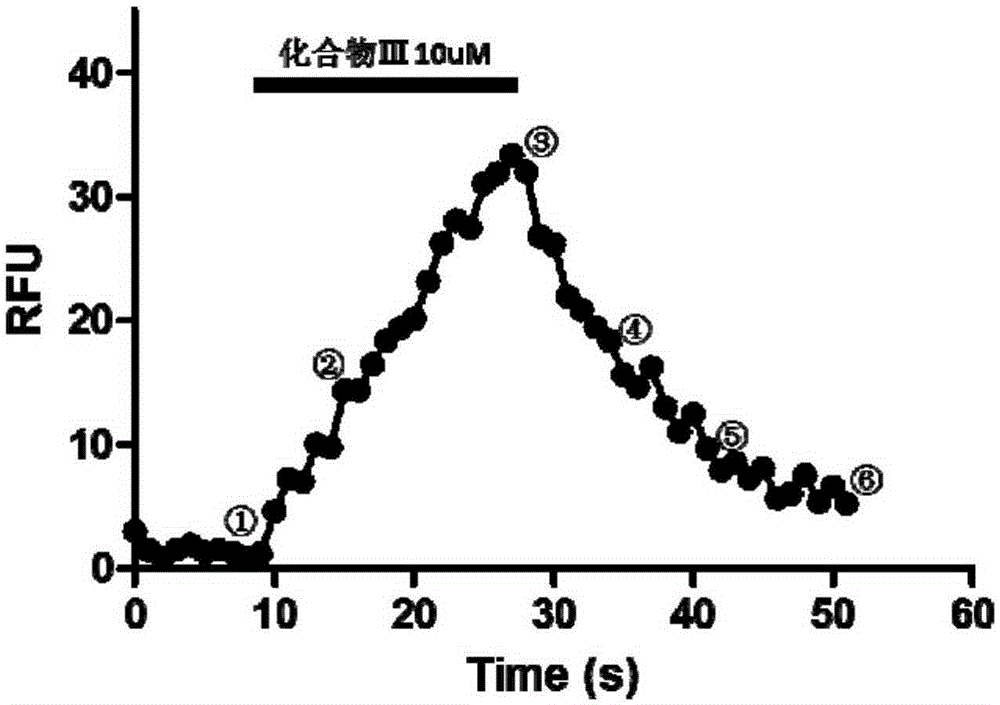
[0047]Take 1 equivalent of compound I and 1 equivalent of R=(CH 2 ) 5 Compound II, dissolved in DMF, was added with 1 equivalent of HOBT, 1 equivalent of DCC, and 0.1 equivalent of DMAP, and stirred at room temperature for 24 hours. The organic solvent was removed, and column chromatography was eluted with dichloromethane:methanol=10:1 to obtain the corresponding compound III. It was confirmed by nuclear magnetic spectrum that it was consistent with the target product, and its hydrogen nuclear magnetic resonance spectrum was assigned as follows: 1 HNMR (500MHz, DMSO-d 6 ): δ9.54(s,1H),8.81(s,1H),8.50(t,1H,J=6Hz),8.16(t,1H,J=6Hz),6.79(d,1H,J=1.5Hz ),6.68(d,1H,J=8Hz),6.62(dd,1H,J=8Hz,J=2Hz),6.39(d,1H,J=9Hz),4.13(d,2H,J=5.5Hz) ,3.72(s,3H),3.44(s,2H),2.13(t,2H,J=7.5Hz),1.68(dt,2H),1.57(dt,2H),1.36(d,2H,J=6Hz ). The fluorescence excitation wavelength of the compound in methanol solvent is 466nm, and the emission wavelength is 528nm. In biological activity assays, the compoun...
Embodiment 2
[0049] Take 1 equivalent of compound I and 1 equivalent of R=(CH 2 ) 11 Compound II, dissolved in DMF, was added with 1 equivalent of HOBT, 1 equivalent of DCC, and 0.1 equivalent of DMAP, and stirred at room temperature for 24 hours. The organic solvent was removed, and column chromatography was eluted with dichloromethane:methanol=10:1 to obtain the corresponding compound III. It was confirmed by nuclear magnetic spectrum that it was consistent with the target product, and its hydrogen nuclear magnetic resonance spectrum was assigned as follows: 1 HNMR (500MHz, DMSO-d 6 )δ9.54(s,1H),8.80(s,1H),8.51(d,1H,J=8.5Hz),8.14(t,1H,J=6Hz),6.79(d,1H,J=2Hz) ,6.68(d,1H,J=8Hz),6.62(dd,1H,J=8Hz,J=2Hz),6.40(d,1H,J=9Hz),4.13(d,2H,J=6Hz),3.72 (s,3H),3.45(s,2H),2.09(t,2H,J=7Hz),1.67(dt,2H),1.49(m,2H),1.36(m,2H),1.26(m,12H ). The fluorescence excitation wavelength of the compound in methanol solvent is 467nm, and the emission wavelength is 528nm. In the biological activity test, the comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com