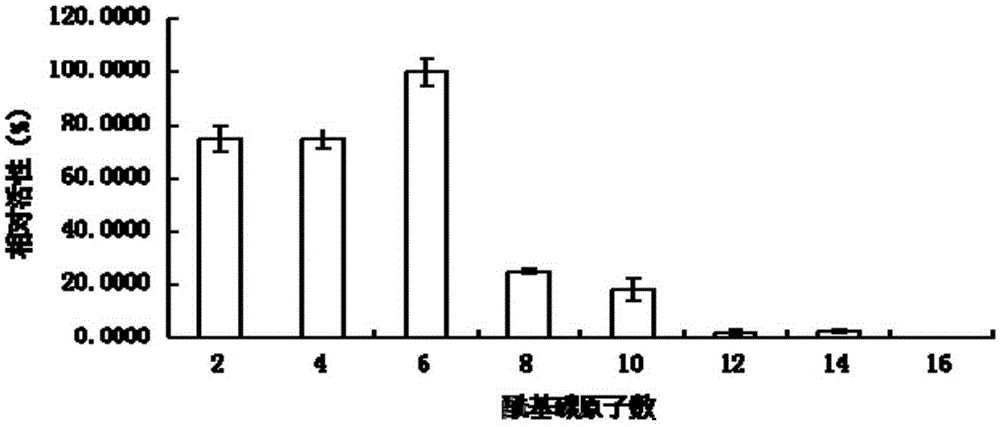
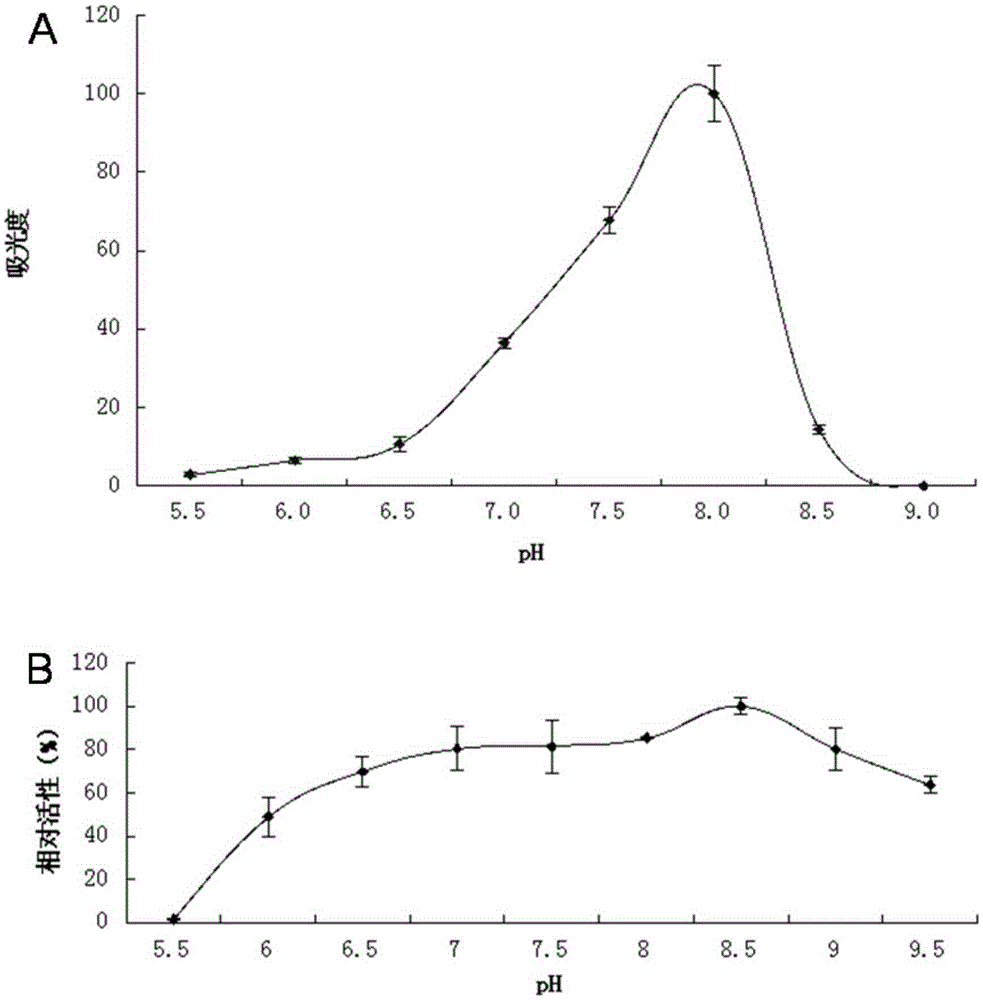
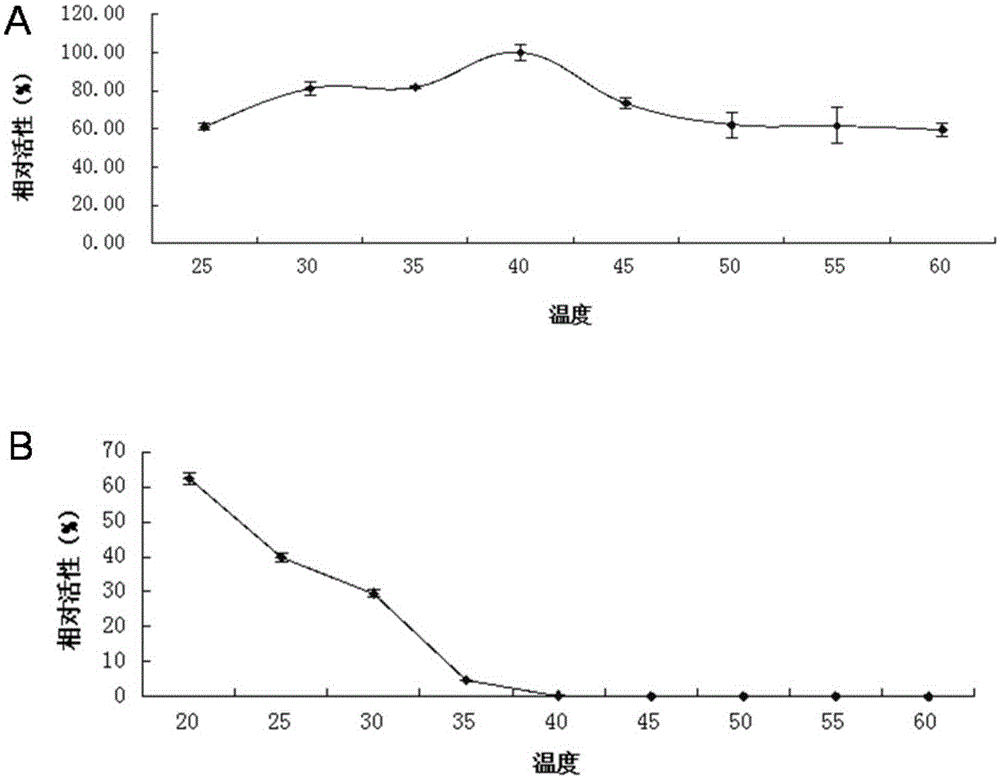
Lipase L-1 and coding gene and application thereof
A lipase gene and lipase technology are applied to lipase L-1 and its encoding gene and application fields, and can solve the problems of production technology restriction, high lipase price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Lipase gene 1-1 primer design and open reading frame boundary determination
[0027] Genomic DNA of actinomycetes (Streptomycessp.) SCSIO13580 was extracted, and after being verified by 16SrRNA, it was submitted to Shanghai Meiji Biotechnology Co., Ltd. for sequencing. Use bioinformatics means to annotate the genome, analyze the lipase gene wherein, and determine the open reading frame of lipase gene 1-1 wherein, its nucleotide sequence is as shown in SEQIDNO.1, full length is 1005bp (from start codon to stop codon), the amino acid sequence of lipase L-1 encoded by it is as shown in SEQ ID NO.2, a total of 334 amino acids; The similarity is 51%. According to the lipase gene 1-1 sequence obtained by analysis, design full-length amplification primers as follows: Forward primer: 5'-CAC GGATCC ATGAGGATCGATCCGCCCG-3′, the underlined part is the BamHI restriction site; reverse primer: 5′-CAT CTCGAG TTAGGCGGGCTGTGGGGTC-3', the underlined part is the XhoI restri...
Embodiment 2
[0028] Embodiment 2: Cloning and vector construction of lipase gene 1-1
[0029] 2.1PCR amplification
[0030] The primer designed in Example 1 (forward primer 5'-CAC GGATCC ATGAGGATCGATCCGCCCG-3′, reverse primer 5′-CAT CTCGAG TTAGGCGGGCTGTGGGGTC-3') was sent to Shanghai Bioengineering Co., Ltd. to synthesize primers, and the synthesized primers were diluted to 10 μM with TE, and the total DNA of actinomycetes (Streptomycesssp.) SCSIO13580 was extracted as a DNA template, and the reaction system shown in Table 1 was established:
[0031] Table 1 PCR reaction system
[0032]
[0033] Amplify lipase gene l-1 using the following PCR amplification program: a. Denaturation at 95°C for 5 min; b. Denaturation at 95°C for 1 min, annealing at 55-65°C for 0.5 min, extension at 72°C for 1 min and 20 s, for 30 cycles; c.72 Extend at ℃ for 10min, then cool to 10℃.
[0034] The PCR product was electrophoresed in 1% agarose gel for 20 min at 120V, and observed in a gel imaging syste...
Embodiment 3
[0045] Example 3: High expression of lipase L-1 in Escherichia coli BL21 (DE3)
[0046] 3.1 Preparation of Escherichia coli BL21(DE3) Competent Cells
[0047] a. Inoculate Escherichia coli BL21(DE3) into 5mL LB liquid medium, shake overnight at 37°C, 250rpm;
[0048] b. Inoculate the Escherichia coli BL21 (DE3) bacterium solution after overnight shaking culture into LB shake flasks at an inoculation rate of 1% volume ratio, and shake at 37° C. for 3 hours (≥ 300 rpm) to obtain the original culture;
[0049] c. Rapidly cool the shake flask to 0°C in ice water, divide the original culture into ice-precooled centrifuge tubes (50 mL), and place on ice for several minutes;
[0050] d. Centrifuge at 4000rpm for 10 minutes at 4°C to recover the cells, and dry the residual liquid in air (rapidly);
[0051] e. 10mL of 0.1M CaCl pre-cooled on ice 2 Resuspend the cells and centrifuge at 4000rpm for 10min at 4°C to recover the cells;
[0052] f. 10mL of 0.1M CaCl 2 Resuspend the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com