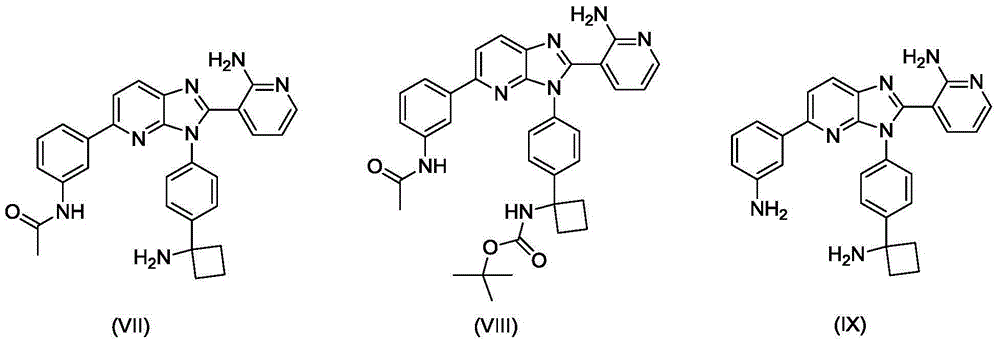
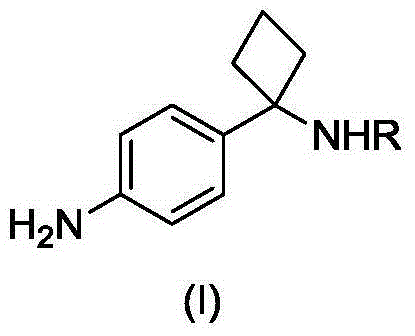
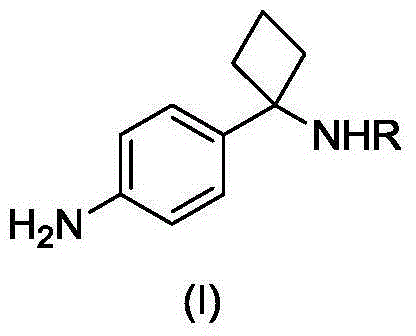
Method for preparing key intermediate of PKB/Akt inhibitor
A technology of solvent and reagent, which is applied in the field of preparation of N-substituted-1-cyclobutylamine, can solve problems such as danger, low reaction yield, unsuitability for industrial production, etc., achieves low environmental pollution, high product purity, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment one: the synthesis of 1-(4-nitrophenyl) cyclobutanecarbonitrile (Ⅲ)
[0055] At room temperature, add 600mL methanesulfonic acid into the reaction flask, slowly add 326gMg(NO 3 ) 2 . 6H 2 O, stirred for 2h, slowly added 200g of 1-phenylcyclobutanecarbonitrile, then stirred at room temperature for 2h, HPLC showed that the content of the raw material was less than 1%. Poured into 2L of water, stirred for 2 hours, a large amount of solids were precipitated, filtered by suction and dried to obtain 250g of light yellow solids with a yield of 97% and a purity of more than 98%. 1 HNMR (400MHz, CDCl 3 )δ2.13-2.30(m,1H),2.50-2.61(m,1H),2.65-2.73(m,2H),2.90-3.00(m,2H),7.65(d,J=8.8Hz,2H) , 8.31 (d, J=8.8Hz, 2H).
Embodiment 2
[0056] Embodiment two: the synthesis of 1-(4-nitrophenyl) cyclobutanecarboxamide (Ⅳ)
[0057] At room temperature, 202g of 1-(4-nitrophenyl)cyclobutanecarbonitrile and 600mL of 10% sodium hydroxide aqueous solution were added to the reaction flask, 500mL of hydrogen peroxide was added, and stirred at room temperature for 24h. HPLC showed that the content of the raw material was less than 1%. Suction filtration and drying yielded 218 g of a light yellow solid with a yield of 99% and a purity greater than 98%. 1 HNMR (400MHz, CDCl 3 )δ1.90-1.96(m,1H),2.15-2.21(m,1H),2.48-2.55(m,2H),2.85-2.88(m,2H),5.18(br,1H),5.53(br, 1H), 7.49 (d, J=8.8Hz, 2H), 8.22 (d, J=8.8Hz, 2H).
Embodiment 3
[0058] Embodiment three: the synthesis of 1-(4-nitrophenyl) cyclobutanecarboxamide (Ⅳ)
[0059] At room temperature, add 202g of 1-(4-nitrophenyl)cyclobutanecarbonitrile into 1L of dimethyl sulfoxide, add 276g of potassium carbonate, add 500mL of hydrogen peroxide, stir at room temperature for 3h, HPLC shows that the content of raw materials is less than 1% . After adding 2L of water, a large amount of solid was precipitated, which was filtered by suction and dried to obtain 209 g of a light yellow solid with a yield of 95% and a purity of more than 98%. 1 HNMR (400MHz, CDCl 3 )δ1.90-1.96(m,1H),2.15-2.21(m,1H),2.48-2.55(m,2H),2.85-2.88(m,2H),5.18(br,1H),5.53(br, 1H), 7.49 (d, J=8.8Hz, 2H), 8.22 (d, J=8.8Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com