Determining method for phosphorylation level of calpain
A technology of phosphorylation level and calpain, which is applied in the biological field, can solve the problems of cumbersome operation process, inactivation of calpain, and great harm to human body, and achieve the effect of less muscle sample, simple operation process and less potential harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
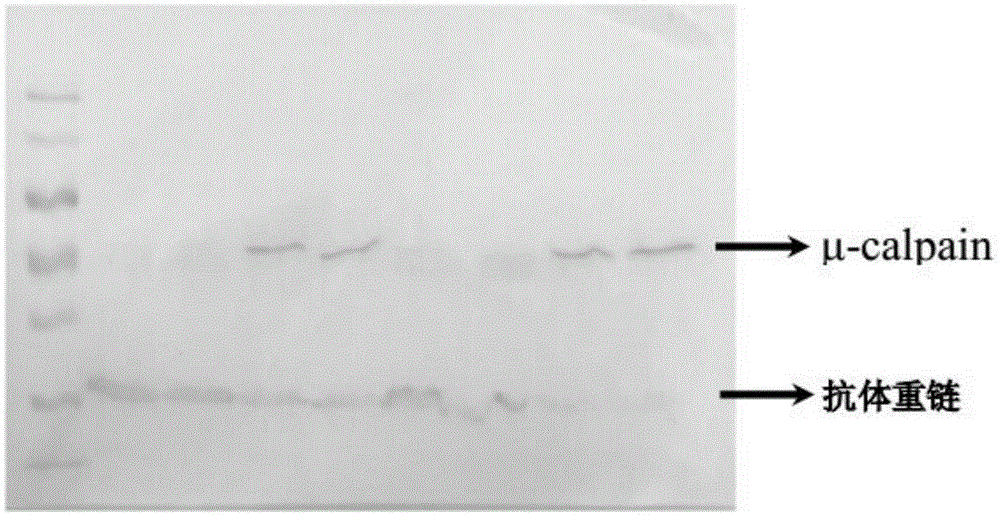
[0073] a) Take part of the longissimus dorsi muscle samples stored at -80°C to prepare tissue lysate, and determine the protein concentration by BCA method;
[0074] b) SDS-PAGE electrophoresis: use 8% separating gel and 4% stacking gel (Acr:Bis=37.5:1), add 50 μg protein sample to each well of the gel; the initial parameter of electrophoresis is set to 70V, when the protein strip After the band completely enters the separation gel, set the voltage to 110V and continue the electrophoresis until the bromophenol blue dye migrates to the edge of the gel bottom and ends the electrophoresis;
[0075] c) Western blotting (IB): remove the gel plate after electrophoresis, put the gel into the transfer buffer to balance; cut the PVDF membrane to the size of the gel, activate it with pure methanol for 15 seconds, and use the prepared transfer membrane exclusively Put the filter paper together in the transfer buffer to balance; use the wet transfer method, ice bath at 100V for 100 minute...
Embodiment 2
[0081] a) Take part of the longissimus dorsi muscle samples stored at -80°C to prepare tissue lysate, and determine the protein concentration by BCA method;
[0082] b) SDS-PAGE electrophoresis: use 8% separating gel and 4% stacking gel (Acr:Bis=37.5:1), add 50 μg protein sample to each well of the gel; the initial parameter of electrophoresis is set to 70V, when the protein strip After the band completely enters the separation gel, set the voltage to 110V and continue the electrophoresis until the bromophenol blue dye migrates to the edge of the gel bottom and ends the electrophoresis;
[0083] c) Western blotting (IB): remove the gel plate after electrophoresis, put the gel into the transfer buffer to balance; cut the PVDF membrane to the size of the gel, activate it with pure methanol for 15 seconds, and use the prepared transfer membrane exclusively Put the filter paper together in the transfer buffer to balance; use the wet transfer method, ice bath at 100V for 100 minute...
Embodiment 3
[0089] a) Take out the muscle sample stored at -80°C, weigh 0.5g in a frozen state, cut it into small pieces and put it into a centrifuge tube;
[0090] b) Add 6 times the volume of sarcoplasmic extract (100 mM Trisbase, 10 mM EDTA, 0.05% mercaptoethanol, pH 8.3), and homogenize on ice for 30 s (every 10 s of homogenization is separated by 20 s);
[0091] c) Centrifuge the homogenate at 10,000×g in a centrifuge at a low temperature of 4°C for 35 minutes;
[0092] d) After skimming the upper fat layer, take the supernatant and subpackage;
[0093] e) Protein quantification: BCA method, store at -80°C for later use after measuring the concentration;
[0094] f) SDS-PAGE electrophoresis: use 8% separating gel and 4% stacking gel (Acr:Bis=37.5:1), add 50 μg protein sample to each well of the gel; the initial parameter of electrophoresis is set to 70V, when the protein strip After the band completely enters the separation gel, set the voltage to 110V and continue the electrophoresi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com