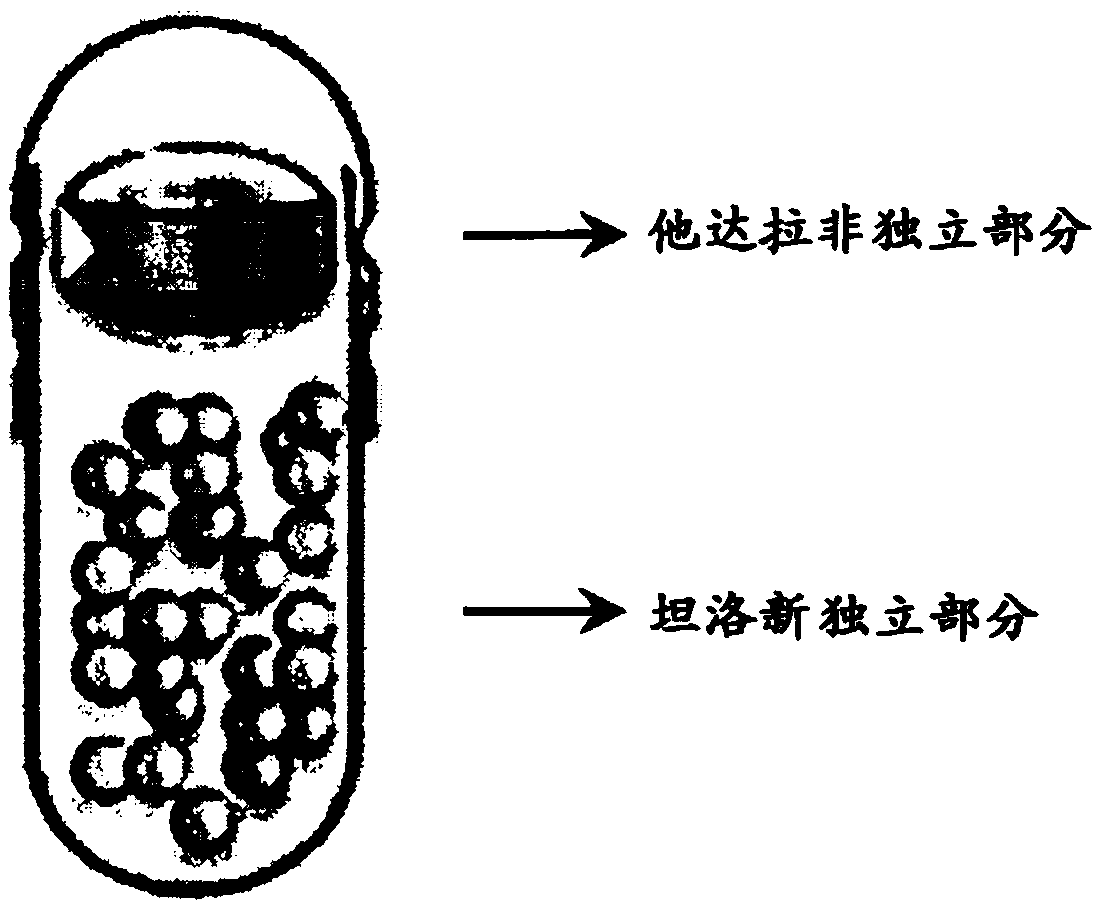
Pharmaceutical capsule composite formulation comprising tadalafil and tamsulosin
A compound preparation, the technology of tadalafil, is applied in the directions of pharmaceutical combinations, medical preparations without active ingredients, medical preparations containing active ingredients, etc. Problems such as poor stability and difficulty in developing fixed compound formulations to achieve the effects of excellent product stability, high productivity, and excellent dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
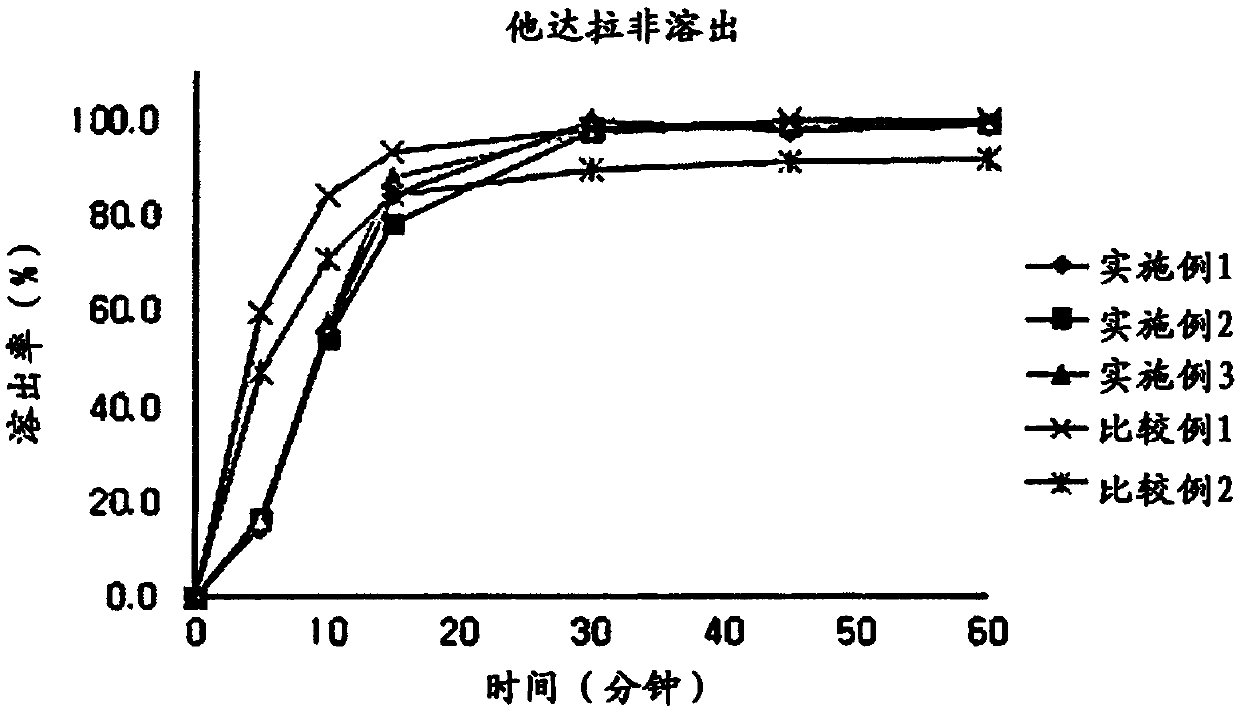
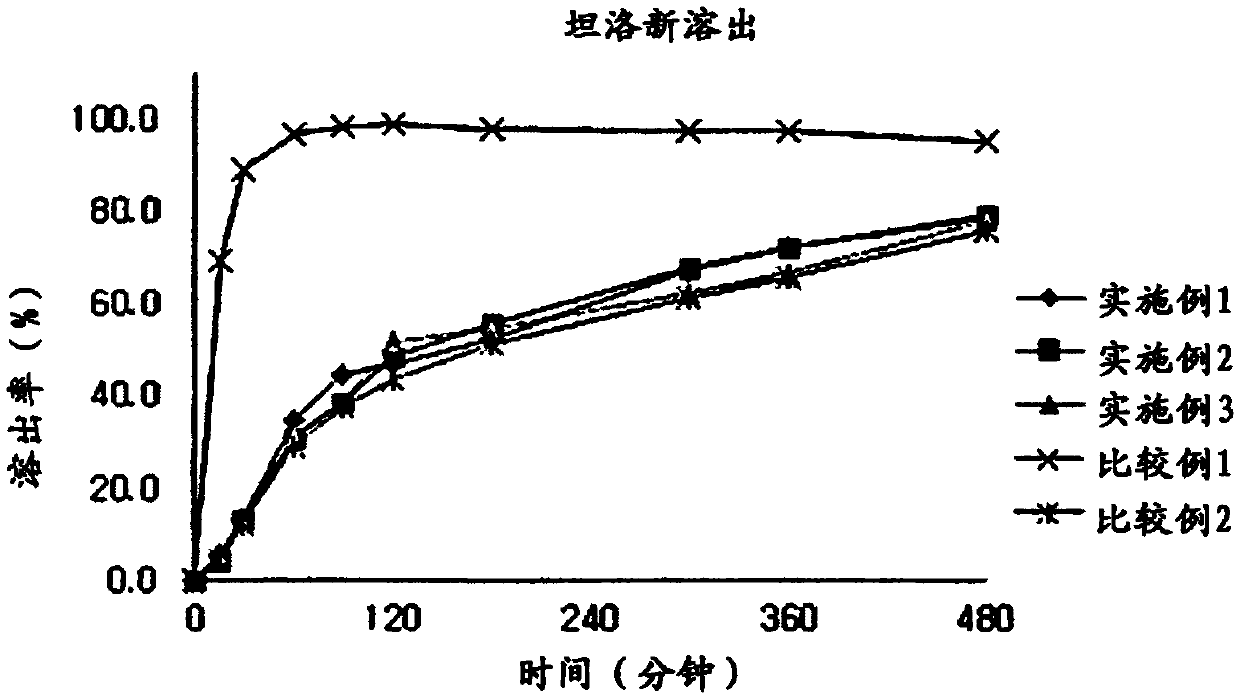
Embodiment 1
[0050] Embodiment 1: Capsule compound preparation I
[0051]
[0052]
[0053]
[0054]
[0055]
[0056] The ingredients in powder form corresponding to individual fractions of tadalafil were mixed and the mixture was compressed into tablets by a round punch with a diameter of 5.5 mm. The tadalafil tablet of gained is coated with solution (that is, Yellow (Colorcon Ltd.) solution in purified water) for coating.
[0057] In addition, ingredients corresponding to separate parts of tamsulosin were mixed in powder form, and the mixture was prepared into granules. The resulting tamsulosin granules are coated with an inner coating solution (i.e., a solution of povidone, propylene glycol and polyvinyl acetate in water), and then coated with an outer coating solution (i.e., methacrylate-ethyl acrylate copolymer and triacetin in water) for further coating.
[0058] The thus coated tadalafil tablets and tamsulosin granules were filled into hard capsule No.1 having hypr...
Embodiment 2
[0059] Embodiment 2: capsule compound preparation II
[0060] A capsule compound formulation containing 5 mg of tadalafil and 0.2 mg of tamsulosin hydrochloride was prepared in the same manner as in Example 1, except that the hard capsule used had pullulan as the capsule material.
Embodiment 3
[0061] Embodiment 3: capsule compound preparation III
[0062] A capsule composite formulation containing 5 mg of tadalafil and 0.2 mg of tamsulosin hydrochloride was prepared in the same manner as in Example 1, except that the hard capsule used had gelatin as the capsule material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com