Dual-core chromium catalyst for ethylene oligomerization and preparation method thereof
A dual-nuclear chromium catalyst, ethylene oligomerization technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve harsh reaction conditions, poor catalyst activity, product Low purity and other problems, to achieve the effect of low polymer product content, long catalyst life and high catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
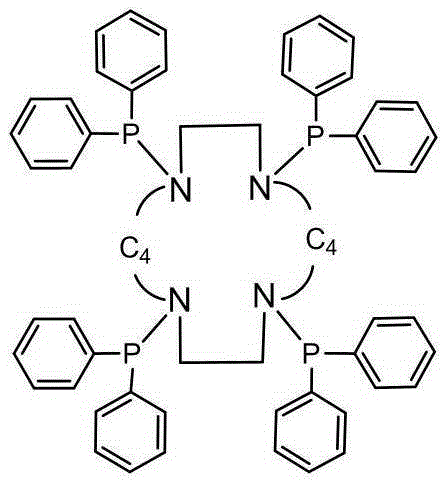
[0032] 1. Preparation of 1,4,7,10-tetrakis(diphenylphosphineamine)-1,4,7,10-tetraazacyclododecane ligand (C 56 h 56 N 4 P 4 )
[0033] The structure of the ligand 1,4,7,10-tetrakis(diphenylphosphineamine)-1,4,7,10-tetraazacyclododecane is shown in the following structural formula:
[0034]
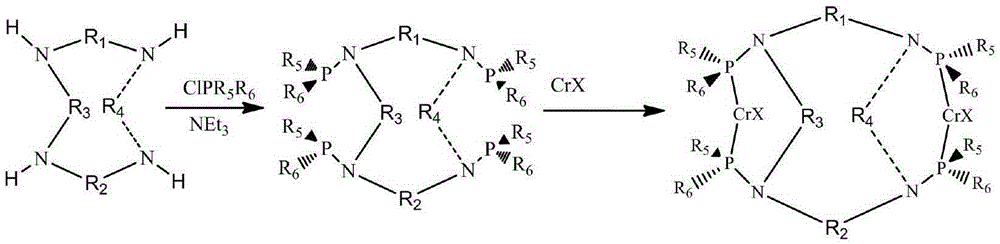
[0035] The preparation method is as follows:
[0036] in the N 2 Add dehydrated dichloromethane (20mL), triethylamine (3.75mL), and diphenylphosphine chloride (1.326mL, 7.2mmol) into a fully replaced stirred 100mL reactor, cool to 0°C, and slowly 1,4,7,10-Tetraazacyclododecane (1.75 mmol) was added. After stirring for 30 minutes, the reaction was continued at room temperature for 12 hours. Filtration and drying afforded the product (1.18 g, 74.2%).
[0037] 1 HNMR (400MHz, CDCl 3 ):d=2.935-2.951(m,16H,(CH 2 ) 2 ),7.132-7.249(m,40H,P(Ph) 2 ); 13 C{ 1 H}NMR (100MHz, CDCl 3 ):d=139.82, 139.68, 131.88, 131.68, 128.42-128.12, 77.33-76.70.31 PNMR (121MHz, CDCl 3 )δ: 66.496.
...
Embodiment 2
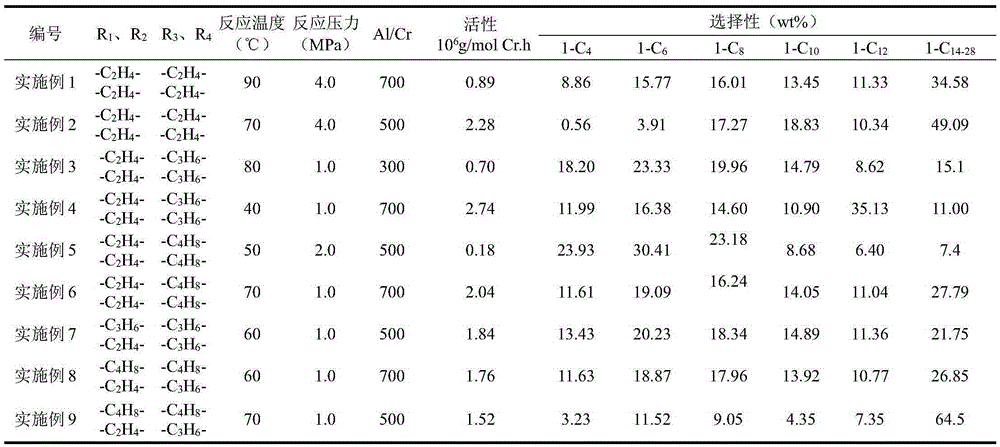
[0043] Same as Example 1, except that the addition amount of 1.4 mol / L MAO in toluene solution is 1.7 mL. (2.4 mmol), 70°C. The catalyst activity is 2.28×10 6 g oligomer / molCr h. The distribution of the oligomerization products is shown in Table 1.
Embodiment 3
[0045] 1. Preparation of 1,5,8,12-tetrakis(diphenylphosphineamine)-1,5,8,12-tetraazacyclotetradecane ligand (C 58 h 60 N 4 P 4 )
[0046] The structure of the ligand 1,5,8,12-tetrakis(diphenylphosphineamine)-1,5,8,12-tetraazacyclotetradecane is shown in the following structural formula:
[0047]
[0048] The preparation method is as follows:
[0049] Add dehydrated dichloromethane (20mL), triethylamine (3.75mL), diphenylphosphorous chloride (1.33mL, 7.2mmol) into a stirred 100mL reactor fully replaced by N2, and cool to 0 °C, 1,5,8,12-tetraazacyclododecane (1.75 mmol) was added slowly. After stirring for 30 minutes, the reaction was continued at room temperature for 12 hours. Filtration and drying gave the product (1.40 g, 85.5%).
[0050] 1 HNMR (400MHz, CDCl 3 ):d=1.436(s,4H,CH 2 ),2.711-2.728(d,8H,NCH 2 CH 2 CH 2 N),3.009-3.035(d,8H,NCH 2 CH 2 N),7.096-7.290(m,40H,P(Ph) 2 ); 13 C{ 1 H}NMR (100MHz, CDCl 3 ):d=139.52, 139.37, 132.08, 131.88, 128.41-128.12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com