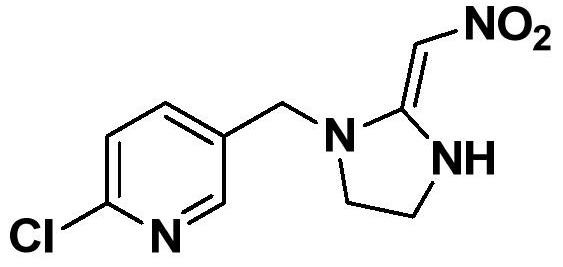
A kind of synthetic method of 2-chloro-5-((2-(nitromethylene)imidazolin-1-yl)methyl)pyridine
A technology of nitromethylene and chloromethylpyridine, which is applied in the field of organic synthesis and can solve problems such as unfavorable industrial production, low atom economy, and poor operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
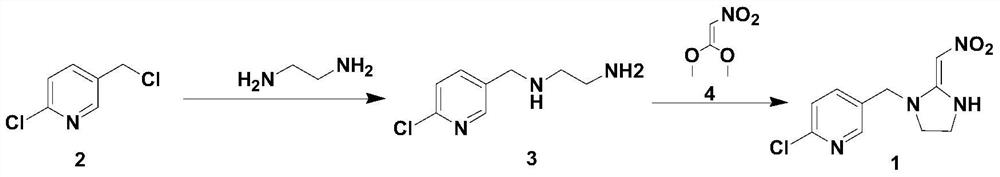
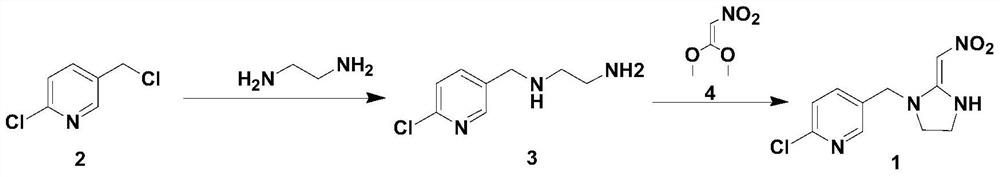
[0028] And, the synthetic method of described 2-chloro-5-((2-(nitromethylene) imidazolin-1-yl) methyl) pyridine comprises the following steps:
[0029] Step 1. Add 2-chloro-5-chloromethylpyridine (2) and a solvent as starting materials into the container, add a phase transfer catalyst and a base, and add ethylenediamine at a temperature of -20°C to 50°C; Then, a substitution reaction was carried out at a temperature of -20°C to 50°C for 3-5 hours to obtain chloropyridine ethylenediamine (3);
[0030] Step ②Heat up to 0°C-35°C, then add 1,1-dimethoxy-2-nitroethylene, heat to reflux, and stir the reaction until the reaction is complete; after-treatment, the target product 2-chloro- 5-((2-(nitromethylene)imidazolin-1-yl)methyl)pyridine.
[0031] In a preferred embodiment, in the above synthesis method, the solvent is selected from any one of the following: water, methanol, ethanol, sec-butanol, tert-butanol, ethylene glycol, acetonitrile, N,N-dimethyl Formamide, dimethyl sulfox...
Embodiment 1
[0041] Add 2-chloro-5-chloromethylpyridine (0.2mol) and toluene 60mL into a 250ml three-necked flask, add tetrabutylammonium bromide (5mmol) and potassium carbonate solid (0.4mol), cool to -20°C, Then add ethylenediamine (1.0mol); then react at -20°C for 5h, and a substitution reaction occurs to obtain chloropyridine ethylenediamine; heat up to 20°C, and then add 1,1-dimethoxy-2-nitroethylene (0.18mol), heated to reflux, and stirred at reflux for 4h. After the reaction is complete, cool to room temperature, filter with suction, recrystallize the filter cake with absolute ethanol, and then dry the resulting crystalline substance to obtain the target product 2-chloro-5-((2-(nitromethylene) Imidazolin-1-yl)methyl)pyridine with a purity of 98.5% and a yield of 76% (based on 2-chloro-5-chloromethylpyridine).
Embodiment 2
[0043] Add 2-chloro-5-chloromethylpyridine (0.2mol) and toluene 60mL in a 250ml three-necked flask, and add dodecyltrimethylammonium chloride (5mmol), add potassium hydroxide solid (0.4mol), Cool down to -15°C, then add ethylenediamine (1.0mol); then react at -15°C for 5 hours, a substitution reaction occurs to obtain chloropyridine ethylenediamine; heat up to 20°C, then add 1,1-dimethoxy -2-Nitroethylene (0.18mol), heated to reflux, and stirred at reflux for 5h. After the reaction is complete, cool to normal temperature, filter with suction, recrystallize the filter cake with absolute ethanol, and then dry the resulting crystalline substance to obtain the target product 2-chloro-5-((2-(nitromethylene) Imidazolin-1-yl)methyl)pyridine with a purity of 98.8% and a yield of 91% (based on 2-chloro-5-chloromethylpyridine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com