Reagent kit for influenza a H3N2 type fluorescent PCR diagnosis and use method thereof
A diagnostic kit, H3N2 technology, applied in the field of medical detection, can solve the problems of poor specificity and low sensitivity, and achieve the effects of excellent detection sensitivity, good treatment, and excellent RNA extraction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
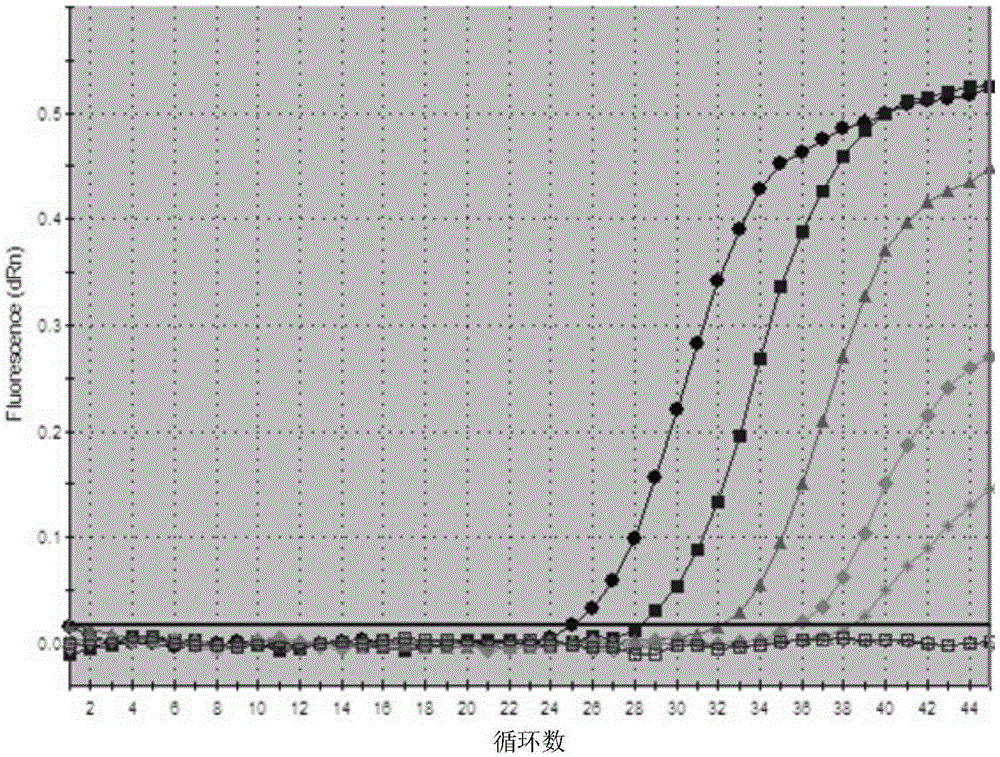
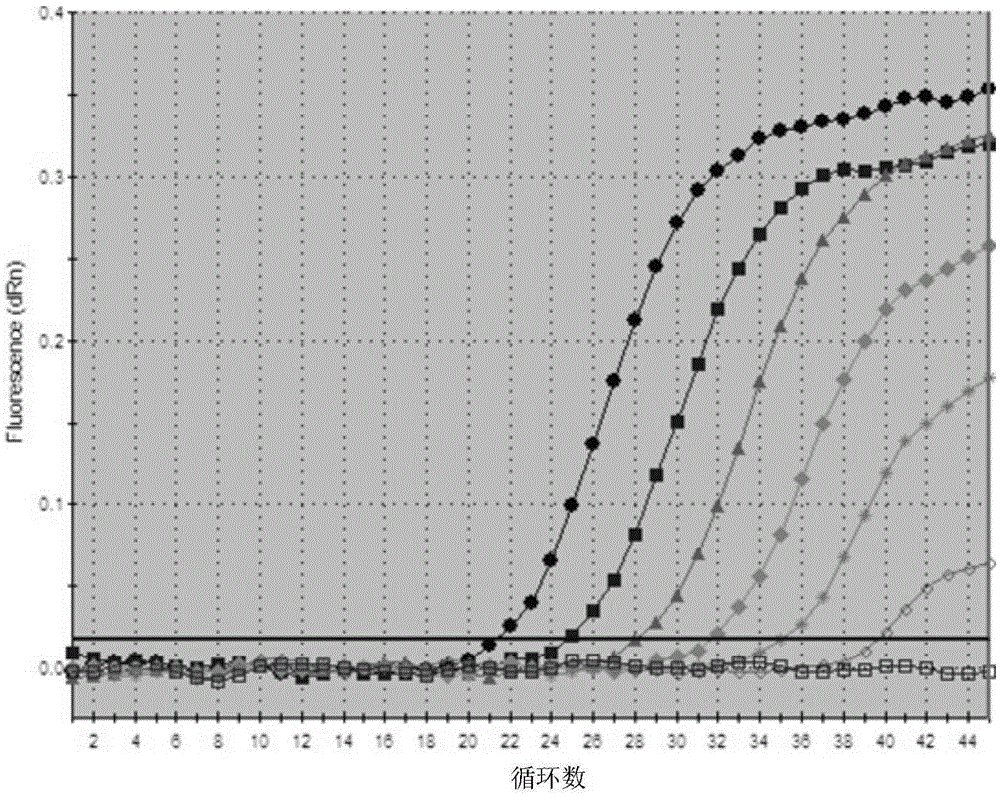
Image
Examples
Embodiment 1
[0030] The present invention provides an influenza A H3N2 fluorescent PCR diagnostic kit. The influenza A H3N2 fluorescent PCR diagnostic kit includes an RNA extraction solution and a PCR reaction solution, wherein the RNA extraction solution includes:
[0031] (1) RNA extraction solution I: composed of sodium dodecyl sulfate with a mass / volume ratio of 0.2% to 1.0%, triton with a volume / volume ratio of 1.0% to 4.0%, 0.2mol / L to 1.0mol / L of guanidine isothiocyanate and 100~400μg / ml magnetic beads;
[0032] (2) RNA extraction solution II: 100-300 mmol / L 4-hydroxyethylpiperazineethanesulfonic acid and 100-300 mmol / L, pH6.5±0.2 sodium chloride;
[0033] (3) RNA extraction solution III: volume / volume ratio of 0.1% to 1.0% triton and 100 to 300 mmol / L of sodium chloride;
[0034] (4) RNA extraction solution IV: mineral oil;
[0035] The PCR reaction solution includes: 0.2 μmol / L~0.4 μmol / L of upstream primers and downstream primers for target polynucleotide amplification and 0.2...
Embodiment 2
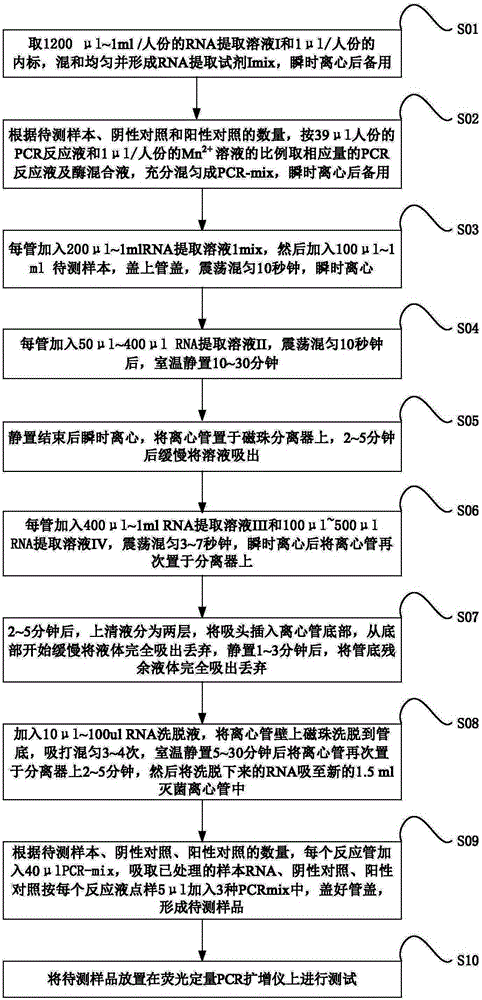
[0058] see figure 1 , shows the flow chart of the use method of the influenza A H3N2 fluorescent PCR diagnostic kit provided by the present invention.
[0059] This embodiment provides the use method of the influenza A H3N2 fluorescent PCR diagnostic kit described in the above embodiment 1 as follows:
[0060] S101: Take 1200 μl~1ml / human portion of RNA extraction solution I and 1 μl / human portion of the internal standard, mix them uniformly to form RNA extraction reagent I-mix, and use it after instantaneous centrifugation;
[0061] S102: According to the number of samples to be tested, negative controls and positive controls, press 39 μl / person of PCR reaction solution and 1 μl / person of Mn 2+ The ratio of the solution is to take the corresponding amount of the PCR reaction solution and the enzyme mixture, fully mix it into a PCR-mix, and centrifuge it briefly for later use;
[0062] S103: Add 200μl~1ml of RNA extraction solution 1-mix to each tube, then add 100μl~1ml of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com