PTD-SMAD7 therapeutics
A nucleic acid molecule, human technology, used in anti-inflammatory agents, non-central analgesics, retro RNA viruses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0243] Example 1: K5.Smad7 mice are resistant to oral mucositis
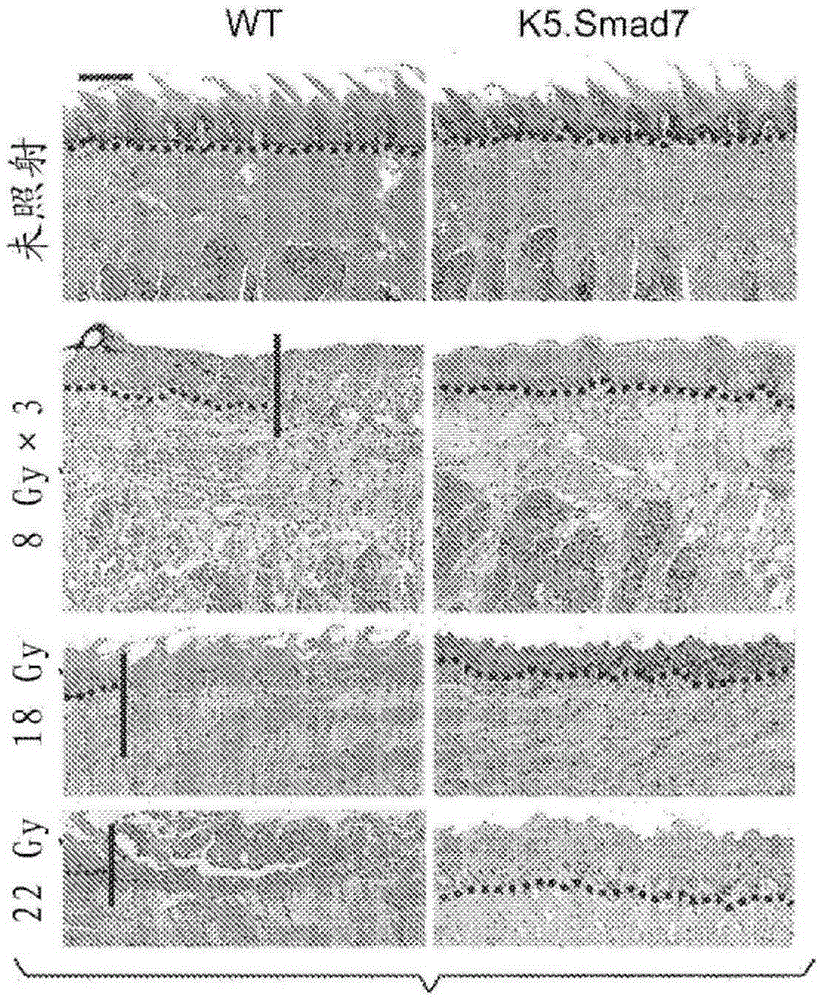
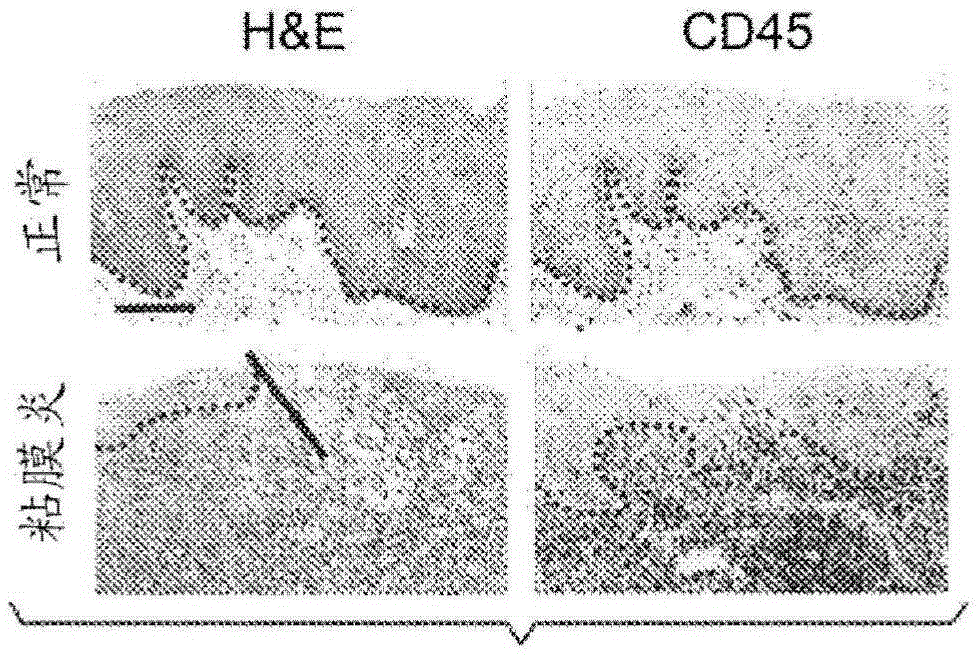
[0244] A transgenic mouse model expressing human Smad7 protein in keratinocytes (K5.Smad7) was generated as previously described (Han et al., Dev. Cell, 11:301-312, 2006). Confirmation of transgene expression in oral epithelium ( Figure 7A -B). Mice were bred into the C57BL / 6 background, and 8-10 week old male and female transgenic mice as well as wild-type littermates were used in the study. These mice showed improved healing of excised skin wounds (Han et al., Am. J. Pathol., 179:1768-1779, 2011) and radiation-induced oral mucositis.
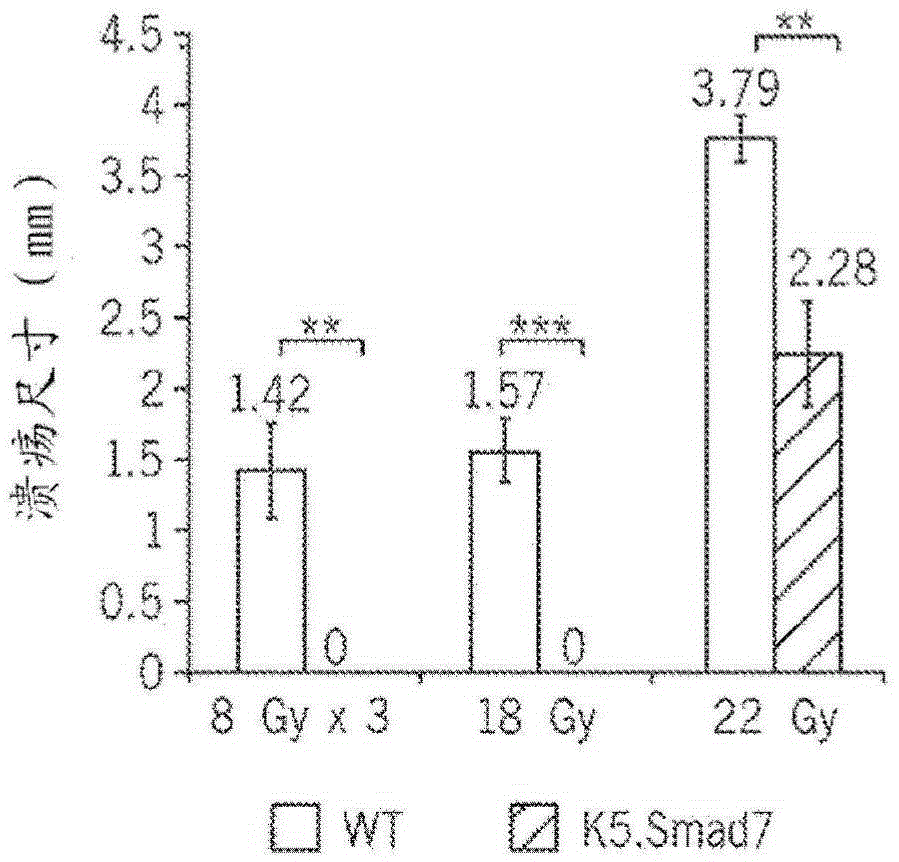
[0245] K5.Smad7 mice and wild-type littermates were exposed to cranial radiation to determine the bioequivalent dose (BED) required to induce oral mucositis in mice. It was determined that 8Gy x 3 (BED = 43.2), a regimen clinically associated with hypofractionated radiation therapy, was the minimum dose required to induce oral mucositis ( Figure 1A -B). To evaluate the ...
Embodiment 2
[0252] Example 2: Rac1 contributes to Smad7-mediated keratinocyte migration
[0253] To determine whether Smad7 contributes to the healing of human oral keratinocytes, Smad7 was knocked down in spontaneously immortalized human oral keratinocytes (NOK-SI). Smad7 knockdown attenuates keratinocyte migration after incision ( Figure 2D and Figure 8A ). Conversely, knocking down TGF-β1 accelerated keratinocyte migration ( Figures 8B-8D), which is consistent with the accelerated wound healing observed in mice lacking TGF-β1 or Smad3.
[0254] To investigate the molecular mechanism associated with Smad7-mediated keratinocyte migration, Rac1, a protein essential for oral wound healing, was examined. Rac1 decreased after Smad7 knockdown ( Figure 2E ). TGF-β1 overexpression in oral mucositis is expected to activate Rac1 via a Smad-independent mechanism. However, despite a 2-fold increase in total Rac1 protein after irradiation, activated Rac1 protein was not significantly al...
Embodiment 3
[0261] Example 3: Tat-Smad7 attenuates radiation-induced oral mucositis
[0262] The ability of the Smad7 transgene to block multiple pathological processes of oral mucositis prompted us to explore whether localized Smad7 delivery could be used to prevent and treat oral mucositis. Because Smad7 is a nuclear protein, localized Smad7 delivery requires allowing rapid entry of Smad7 into cells before saliva washes away the protein. Therefore, recombinant human Smad7 was generated with an N-terminal Tat tag, which allows the protein to rapidly permeate the cell membrane and enter the nucleus. The V5 epitope was added to the C-terminal end of the Tat-Smad7 protein to track Tat-Smad7 cell penetration ( Figures 11A-11D ).
[0263] Tat-Smad7 biological activity was tested using its ability to block Smad2 phosphorylation ( Figure 11C ). A Tat-Cre recombinant protein with the same tag as the control was produced ( Figure 11E -F) and cloned into the pET101-Topo protein expressio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com