Method for synthesizing pterostilbene and derivatives thereof
The technology of a derivative and pterostilbene is applied in the synthesis field of pterostilbene and its derivatives, and can solve the problems of complicated steps, low yield, unsuitable large-scale production of pterostilbene and its derivatives, etc., and achieves simple steps and high yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
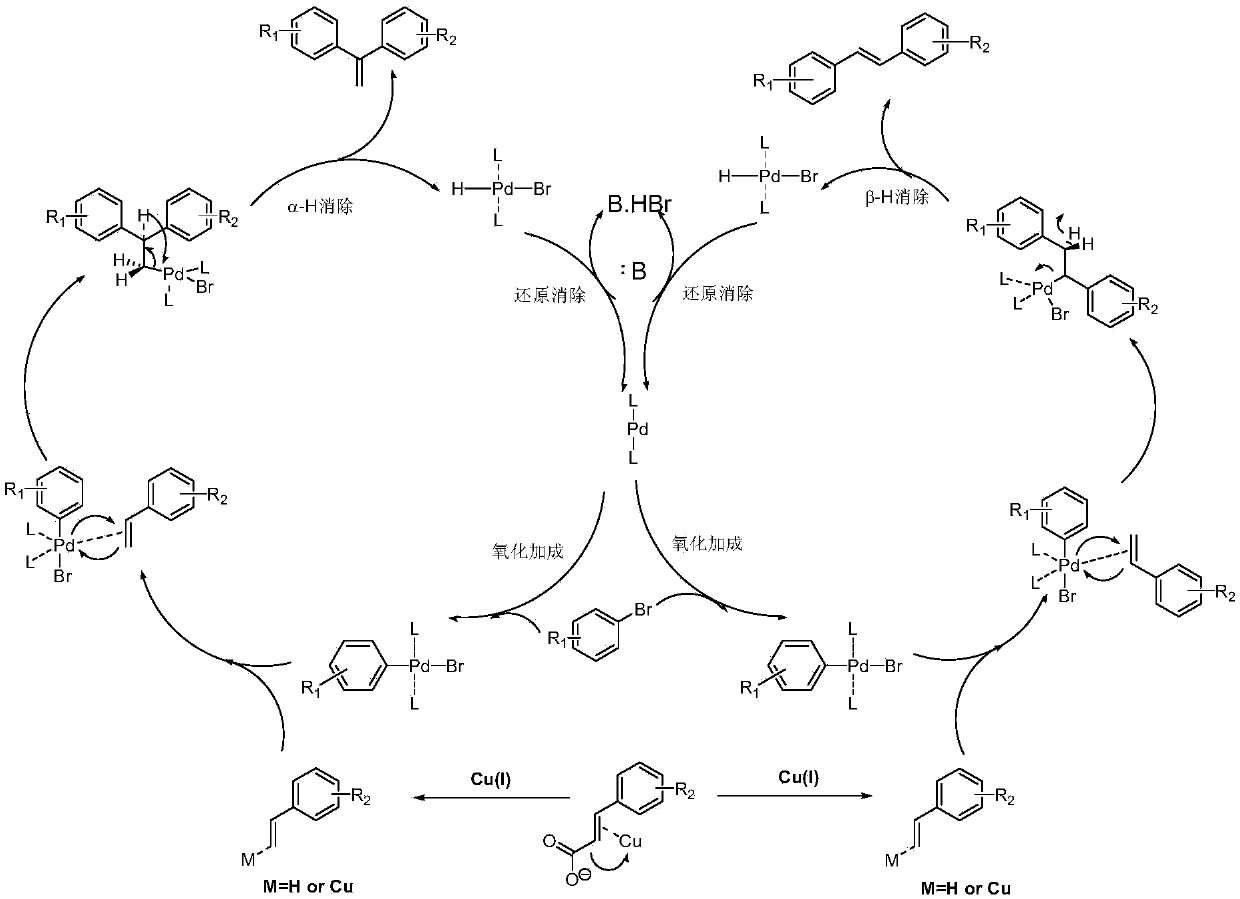
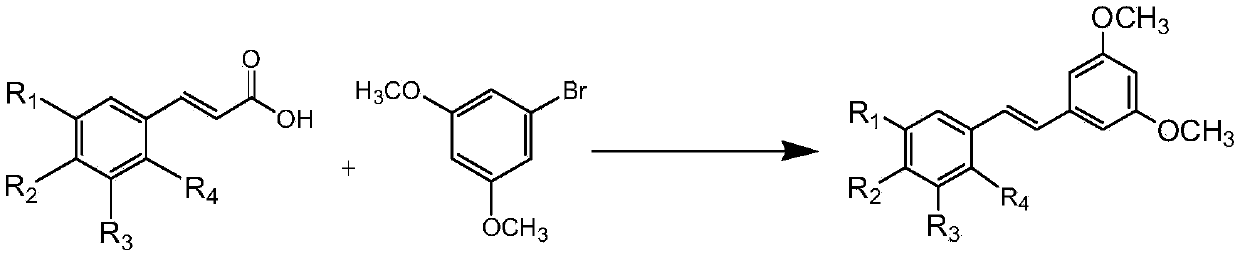
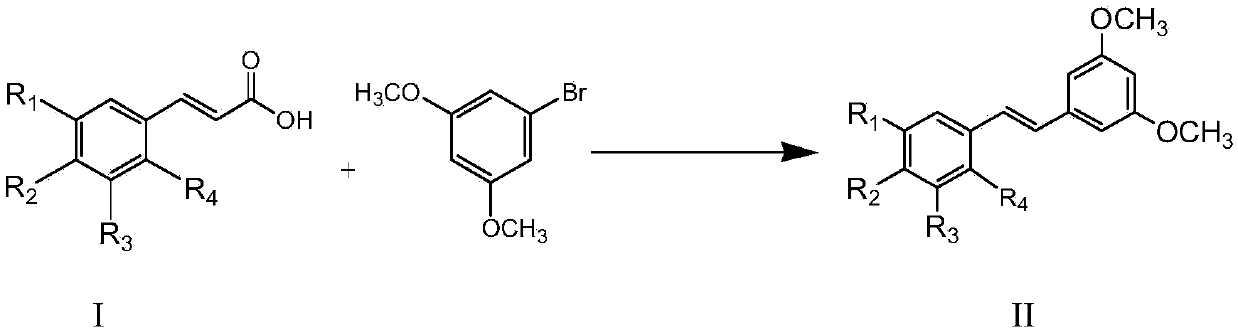
[0026] 7.61mmol of 3,5-dimethoxybromobenzene, 7.61mmol of 3,5-dimethoxycinnamic acid, Cs 2 CO 3 9.14mmol, 1,10-phenanthroline 0.457mmol, triphenylphosphine 0.457mmol, CuCl 1.142mmol, Pd(acac) 2 0.228mmol, then add 7.6ml of reaction solvent N-methyl-1,2-pyrrolidone, and reflux reaction for 3h under the condition of silicone oil bath at about 140°C to obtain the reactant;
[0027] The resulting reactant was lowered to room temperature, extracted with water-dichloroethane with a volume ratio of 1:2, separated, and the organic layer was set aside, and dichloromethane was added to the obtained water layer for extraction, separated, and the organic layer obtained by the two extractions was separated. Combined, anhydrous MgSO 4 Dry, concentrate, and use flash column chromatography (stationary phase is 230-400 mesh silica gel, mobile phase is 10-20% diethyl ether n-hexane solution) to carry out purification, collect the first band, that is, the exo configuration product, and collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com