Trimethyl orthoacetate synthesis method
A technology of trimethyl orthoacetate and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of high processing cost, large steam consumption, no process value, etc., and achieves improved mixing. The effect of reducing the water content of the system and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
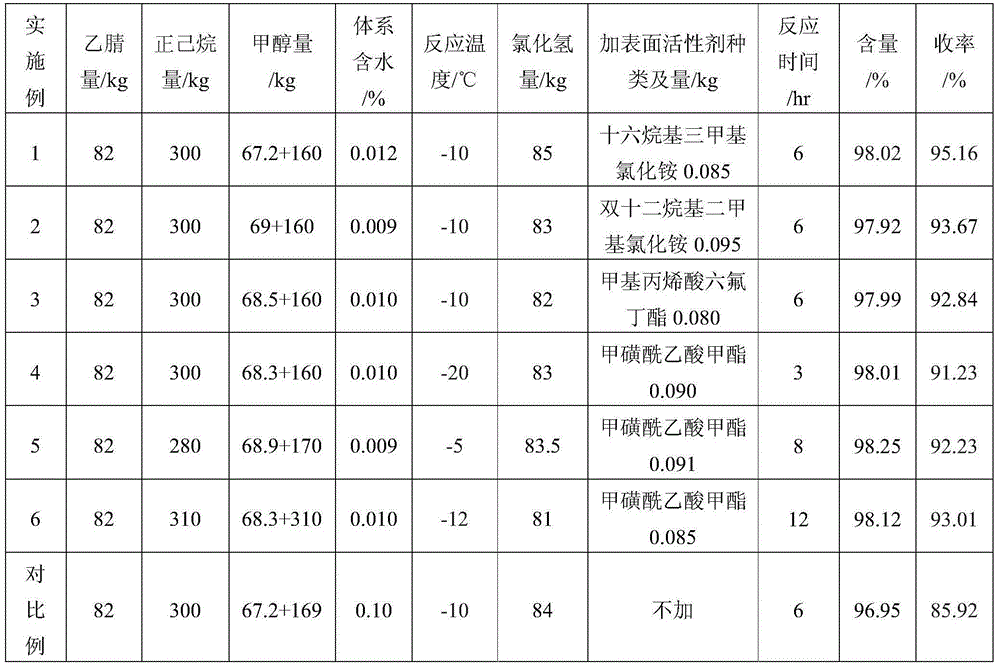
[0025] 1) Put 82kg of acetonitrile, 67.2kg of anhydrous methanol and 300kg of n-hexane in a 1.5m high 1000L enamel reaction kettle equipped with a stirrer and a temperature measuring probe, stir and cool the reaction solution to -10°C, and stir Add 0.085kg of water-absorbing surfactant cetyltrimethylammonium chloride under the state, slowly feed 85kg of dry hydrogen chloride gas, and fully stir and react for 6 hours to form a salt after passing through to obtain alkyliminoacetate hydrochloride Salt.
[0026] 2) Add 160 kg of anhydrous methanol to the alkylimino acetate hydrochloride obtained in step 1), and feed ammonia gas to adjust the pH value of the reaction solution to 5.5-6.0. Keeping it unchanged for 15 minutes, the reaction mixture was stirred at 40°C for 4 hours to complete the alcoholysis, the temperature was lowered and filtered to remove salt, and the filtrate was rectified to obtain 233 kg of trimethyl orthoacetate with a content of 98.02% and a yield of 95.16%. ...
Embodiment 2
[0028] 1) Put 82kg of acetonitrile, 69kg of anhydrous methanol and 300kg of n-hexane in a 1.5m high 1000L enamel reaction kettle equipped with a stirrer and a temperature measuring probe, stir and cool the reaction solution to -10°C, Add 0.095kg of water-absorbing surfactant didodecyldimethylammonium chloride, slowly feed 83kg of dry hydrogen chloride gas, and fully stir and react for 6 hours to form a salt after passing through to obtain alkyliminoacetate hydrochloride. Salt;
[0029] 2) Add 160 kg of anhydrous methanol to the alkylimino acetate hydrochloride obtained in step 1), and feed ammonia gas to adjust the pH value of the reaction solution to 5.5-6.0. Keeping it unchanged for 15 minutes, the reaction mixture was stirred at 40°C for 4 hours to complete the alcoholysis, the temperature was lowered and filtered to remove salt, and the filtrate was rectified to obtain 229.6 kg of trimethyl orthoacetate with a content of 97.92% and a yield of 93.67%.
Embodiment 3
[0031] 1) Put 82kg of acetonitrile, 68.5kg of anhydrous methanol and 300kg of n-hexane in a 1.5m high 1000L enamel reaction kettle equipped with a stirrer and a temperature measuring probe, stir and cool the reaction solution to -10°C, and stir Add 0.080kg of water-absorbing surfactant hexafluorobutyl methacrylate under the state, slowly feed 82kg of dry hydrogen chloride gas, fully stir and react for 6 hours to form a salt after passing through, and obtain alkyliminoacetate hydrochloride;
[0032] 2) Add 160 kg of anhydrous methanol to the alkylimino acetate hydrochloride obtained in step 1), and feed ammonia gas to adjust the pH value of the reaction solution to 5.5-6.0. Keeping it unchanged for 15 minutes, the reaction mixture was stirred at 40°C for 4 hours to complete the alcoholysis, the temperature was lowered and filtered to remove salt, and the filtrate was rectified to obtain 227.4kg of trimethyl orthoacetate with a content of 97.99% and a yield of 92.84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com