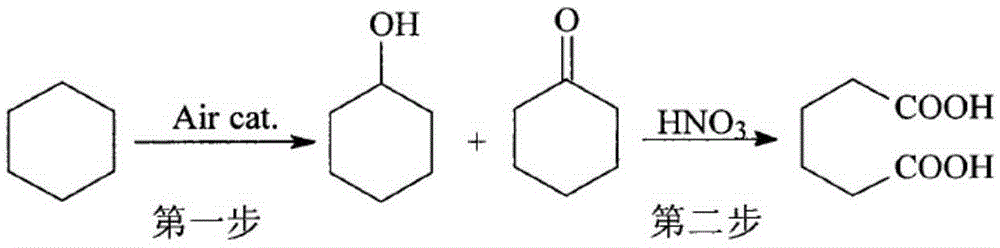
Process method of adipic acid by cyclohexane oxidation
A process method and cyclohexane technology, applied in the field of preparation of adipic acid, can solve the problems of increased production cost, reduced main reaction rate, reduced utilization rate of cyclohexane, etc. rate increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
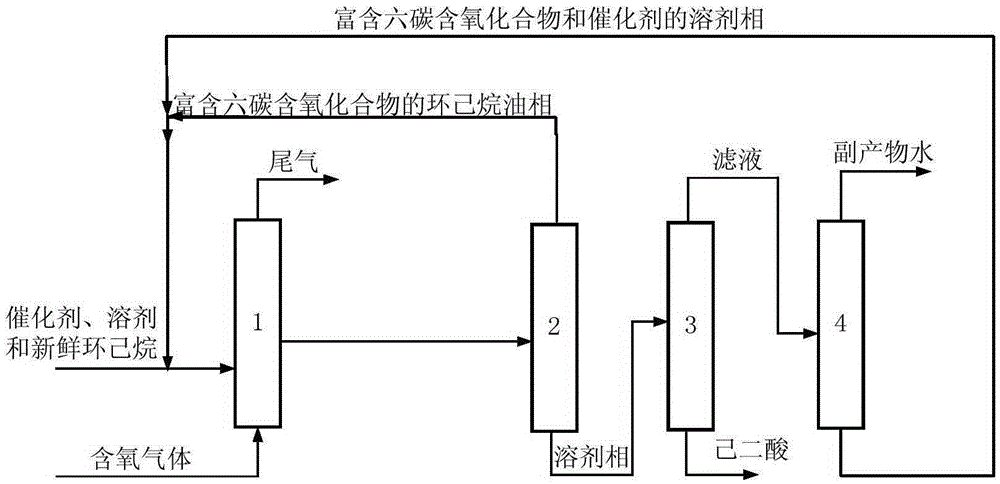
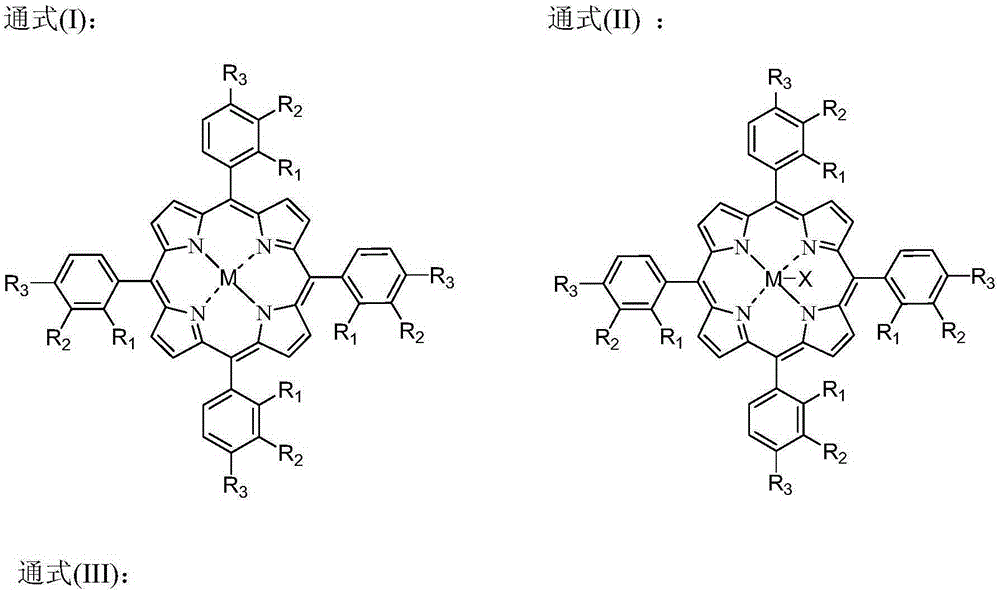
[0075] The metalloporphyrin (R 1 =R 2 =R 3 =H, M=Co) catalyst, the concentration of catalyst relative to cyclohexane is 1 ppm, and the mass ratio of solvent to cyclohexane is 0.084. When the device is in steady state operation, the feed rate of fresh material is 3.2kg / h. The residence time of the reactor based on the flow rate of the liquid phase reactant is 4.0 h. The pressurized pure oxygen is continuously fed into the oxidation reaction kettle to maintain the reaction temperature of the system at 150°C and the reaction pressure at 1.02MPa. The liquid phase at the outlet of the oxidation reactor continuously enters the liquid-liquid stratification system at a temperature of 134.6°C and a pressure of 0.95 MPa to obtain an upper oil phase and a lower solvent phase. The upper oil phase is recycled back to the oxidation reactor to continue the reaction, and the lower solvent phase is subjected to continuous cooling and crystallization in a solid-liquid separation system, and...
Embodiment 2
[0077] The metal phthalocyanine (R 1 = F, R 2 =H, M=Fe) and metalloporphyrins (R 1 =R 2 = F, R 3 =F, M=Co, the catalyzer that the mixture of X=Br) forms, and the concentration of catalyzer relative cyclohexane is 10ppm, and the mass ratio of solvent and cyclohexane is 0.042, and the mass ratio of acetic acid and cyclohexyl acetate is 1: 1. When the device is in steady state operation, the feed rate of fresh material is 11.29kg / h. The residence time of the reactor based on the flow rate of the liquid phase reactants was 3.2 h. Oxygen-enriched air with an oxygen mass concentration of 80% is continuously fed into the oxidation reactor to maintain the system reaction temperature at 142° C. and reaction pressure at 0.92 MPa. The liquid phase at the outlet of the oxidation reactor continuously enters the liquid-liquid stratification system at a temperature of 121.1°C and a pressure of 0.81 MPa to obtain an upper oil phase and a lower solvent phase. The upper oil phase is recy...
Embodiment 3
[0079] The fresh material cyclohexane that enters oxidation reactor and solvent acetic acid+cyclohexyl acetate are dissolved with cobalt isooctanoate and metal phthalocyanine (R with general formula (IV) structure 1 = H, R 2 =CH 3 CH 2 , M=Mn) a mixture of catalysts, the concentration of the catalyst relative to cyclohexane is 100ppm, the mass ratio of solvent to cyclohexane is 0.023, and the mass ratio of acetic acid to cyclohexyl acetate is 2:1. When the device is in steady state operation, the feed rate of fresh material is 30.65kg / h. The residence time of the reactor based on the flow rate of the liquid phase reactants was 2.5 h. The oxygen-enriched air with an oxygen mass concentration of 60% is continuously passed into the oxidation reactor to maintain a system reaction temperature of 146°C and a reaction pressure of 0.97MPa. The liquid phase at the outlet of the oxidation reactor continuously enters the liquid-liquid layered system at a temperature of 127.8°C The pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com