Preparation method for high-purity submicron lithium carbonate
A sub-micron, lithium carbonate technology, applied in lithium carbonate;/acid carbonate and other directions, can solve the problems of large application limitations of production technology, high process energy consumption, long process duration, etc., to ensure The effect of high purity requirements, improving conversion efficiency and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] Hereinafter, embodiments of the present invention will be described in detail. This invention may, however, be embodied in many different forms and should not be construed as limited to the specific embodiments set forth herein. Rather, the embodiments are provided to explain the principles of the invention and its practical application, thereby enabling others skilled in the art to understand the invention for various embodiments and with various modifications as are suited to particular intended uses.
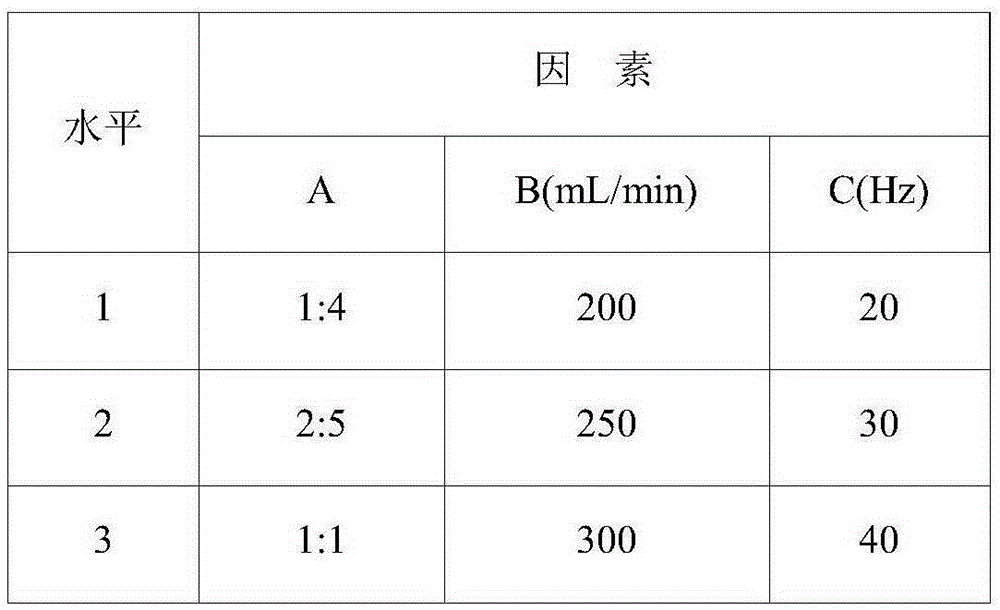
[0023] The invention uses lithium carbonate crude product slurry as a raw material, prepares lithium bicarbonate feed solution through supergravity technology, and then obtains submicron high-purity lithium carbonate through supergravity technology. Through the investigation of three factors such as the concentration of the dispersion liquid, the feed rate, and the speed of the high-speed rotating packed bed in the lithium carbonate precipitation step, a rapid and effi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com