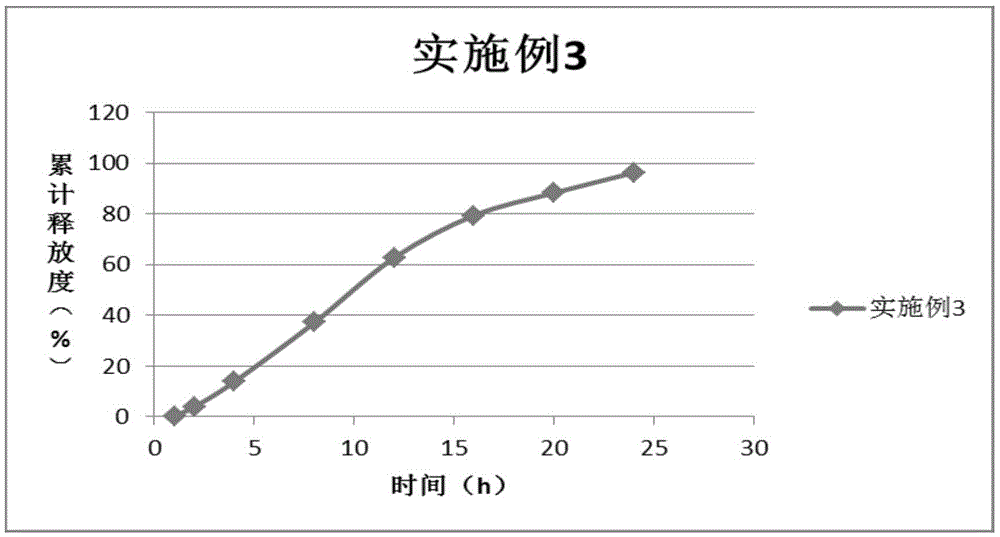
Nifedipine controlled release tablet and preparation method thereof
A technology of nifedipine and tablet cores, applied in the field of nifedipine controlled-release tablets and its preparation, can solve problems such as hindering industrial production and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] The present invention also provides a preparation method of nifedipine controlled-release tablets, comprising the following steps:
[0083] A) mixing 5-45% of nifedipine, 25%-85% of suspending agent, 1-25% of penetration enhancer and 0.5-10% of lubricant to obtain the drug-containing layer material;
[0084] Mixing 45-90% of permeation-enhancing polymer, 5-45% of permeation-enhancing agent, 0.1-4% of coloring agent and 0.1-10% of lubricant to obtain the booster layer material;
[0085] B) compressing the drug-containing layer material and the booster layer material in the step A) to obtain a double-layer tablet core;
[0086] C) Mix the film-forming material, the porogen and the solvent to obtain a coating solution, and use the coating solution to coat the double-layer tablet core obtained in the step B) to obtain a coated tablet. The film-forming The mass ratio of material and porogen is 100: (5-30);
[0087] D) perforating one side of the drug-containing layer of th...
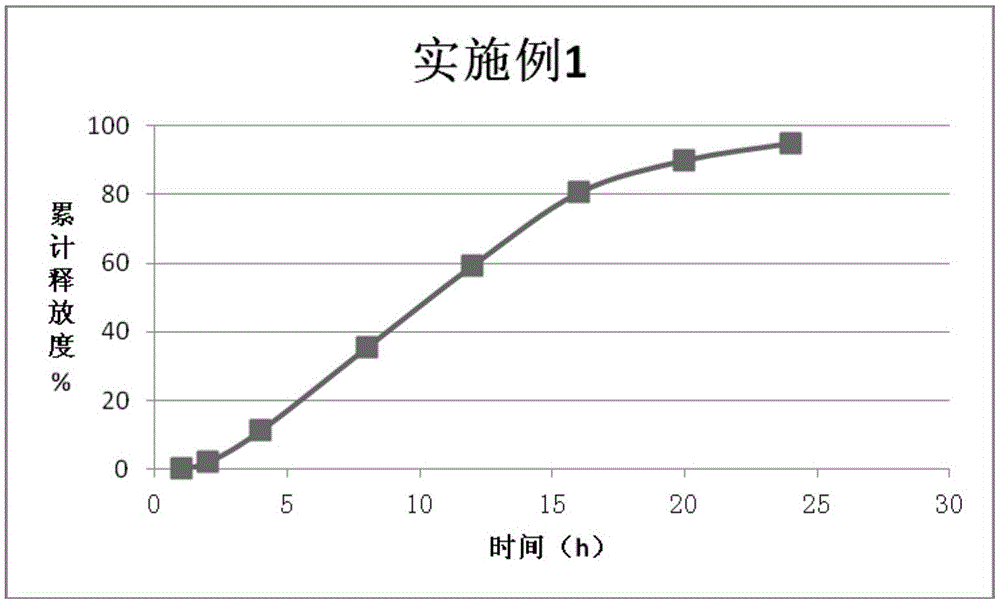
Embodiment 1
[0100] Drug-containing layer prescription (amount per tablet):
[0101]
[0102] Booster layer prescription (amount per tablet):
[0103]
[0104] Controlled release coat prescription (1000 tablets):
[0105]
[0106] Shading layer prescription (1000 tablets):
[0107] Opadry 85G689189g;
[0108] 200g of water.
[0109] According to the above formula, each component of the drug-containing layer and each component of the booster layer were mixed separately for 10 minutes without adding a lubricant, and then mixed separately for 3 minutes after adding a lubricant, and the uniformly mixed drug-containing layer and booster layer were taken. Push layer material, use 9.0mm shallow concave abrasive tool to press double-layer tablet, tablet core hardness is 4kg / mm 2 ;
[0110] The compressed double-layer tablet core is covered with a semi-permeable film, the solid content of the controlled-release coating coating solution is 5%, the tablet bed temperature is 20° C., and ...
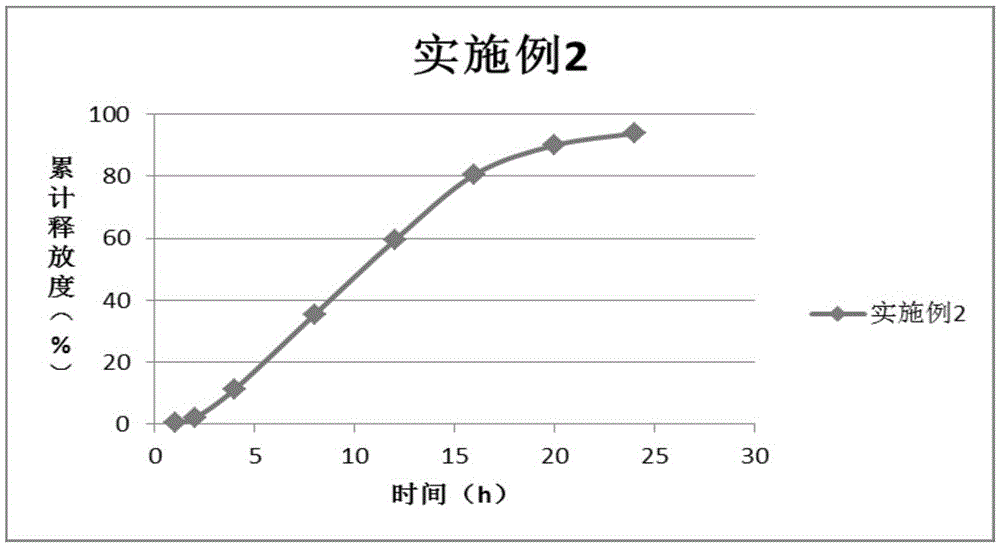
Embodiment 2
[0115] Drug-containing layer prescription (amount per tablet):
[0116]
[0117] Booster layer prescription (amount per tablet):
[0118]
[0119] Controlled release coat prescription (1000 tablets):
[0120] Opadry 19000430g;
[0121] Acetone 1000g;
[0122] 90g of water;
[0123] Shading layer prescription (1000 tablets):
[0124] Opadry 85G689188.5g;
[0125] 190g of water.
[0126] According to the above formula, the components of the drug-containing layer and the components of the booster layer were mixed for 15 minutes without adding lubricant, and then mixed for 5 minutes after adding lubricant, and the uniformly mixed drug-containing layer and booster layer were taken. Push layer material, use 9.0mm shallow concave abrasive tool to press double-layer tablet, tablet core hardness is 6kg / mm 2 ;
[0127] The compressed double-layer tablet core is coated with a semi-permeable film, the solid content of the coating solution of the controlled-release coating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com