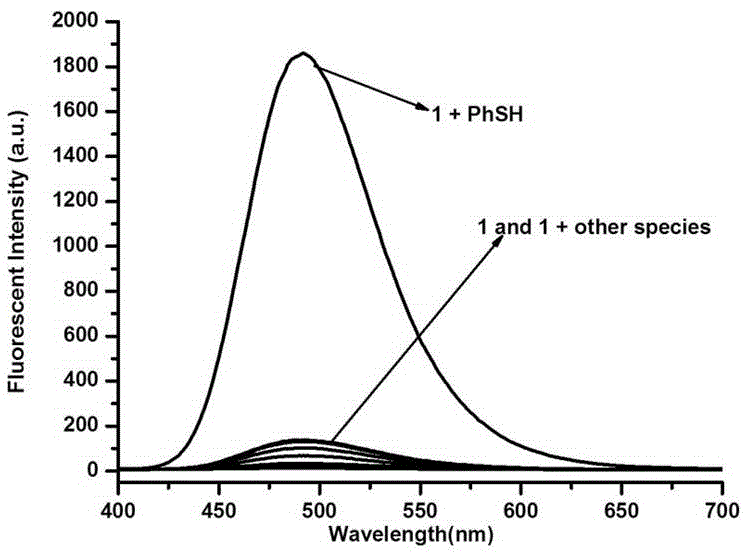
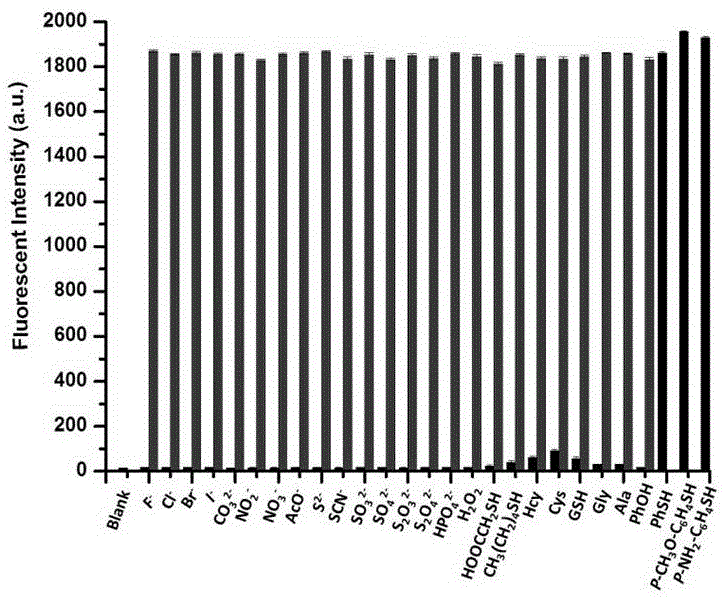
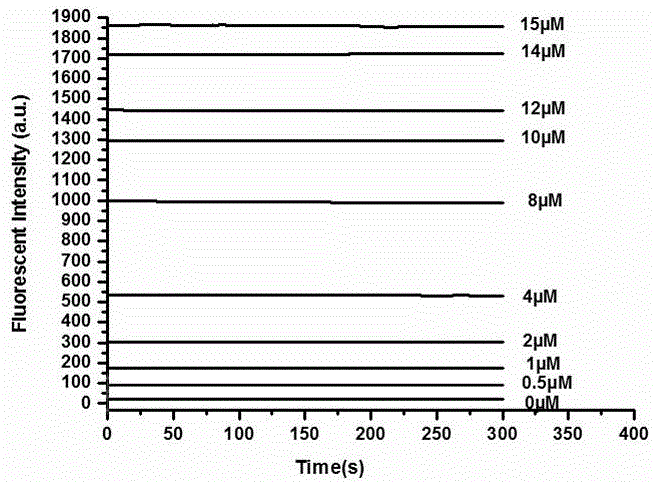
Thiophenol fluorescent probe based on 7-lignocaine-3-hydroxycoumarin structure and preparation method thereof
A technology of hydroxycoumarin and diethylamino, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of inability to selectively distinguish thiophenol and aliphatic thiol, and detect thiophenol It takes a long time to achieve the effect of easy monitoring and control, easy separation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Synthesis of 7-diethylamino-3-hydroxycoumarin. Dissolve 200.0mg (0.86mmol) of 7-diethylamino-3-aminocoumarin in 3mL of 1mol / L hydrochloric acid, heat up to 100°C, follow the reaction by TLC, complete the reaction in 1.5 hours, cool to room temperature, and use 25 % ammonia water was adjusted to neutrality, and a large amount of yellow solids were separated out, extracted three times with 15mL dichloromethane each time, the organic phase was dried with anhydrous sodium sulfate, filtered, the solvent was evaporated under reduced pressure, and the yellow solid was separated and purified by silica gel column chromatography. Green solid 172.7mg, yield 86%. 1 HNMR (400MHz, CDCl 3 ):δ7.21(d,J=8.4Hz,1H),6.99(s,1H),6.63(s,1H),6.56(s,1H),5.80(s,1H)3.40(q,J=6.8 Hz,4H),1.20(t,J=6.8Hz,6H). 13 CNMR (100Hz, CDCl 3 ): δ160.7, 151.3, 147.9, 135.7, 126.8, 115.4, 109.3, 97.6, 44.2, 29.2, 11.9. HRMS (ESI) (C 13 h 15 NO 3 )m / z: calculated for [M+H] + :234.1130.Found[M+H] ...
Embodiment 2
[0019] Example 2: Synthesis of 7-diethylamino-3-hydroxycoumarin. Dissolve 200.0 mg (0.86 mmol) of 7-diethylamino-3-aminocoumarin in 3 mL of hydrochloric acid with a concentration of 1.5 mol / L, heat up to 100 ° C, follow the reaction by TLC, complete the reaction in 1 hour, cool to room temperature, and use 25% ammonia water was adjusted to neutral, and a large amount of yellow solids were precipitated, extracted three times with 15mL dichloromethane each time, the organic phase was dried with anhydrous sodium sulfate, filtered, the solvent was evaporated under reduced pressure, and purified by silica gel column chromatography to obtain Yellow-green solid 184.7mg, yield 92%.
Embodiment 3
[0020] Example 3: Synthesis of 7-diethylamino-3-hydroxycoumarin. Dissolve 200.0mg (0.86mmol) of 7-diethylamino-3-aminocoumarin in 3mL of 1.5mol / L hydrochloric acid, raise the temperature to 100°C, follow the reaction by TLC, complete the reaction in 2 hours, cool to room temperature, and wash with saturated carbonic acid The sodium hydrogen solution was adjusted to be neutral, and a large amount of yellow solids were precipitated, extracted three times with 15mL dichloromethane each time, the organic phase was dried with anhydrous sodium sulfate, filtered, the solvent was evaporated under reduced pressure, and purified by silica gel column chromatography to obtain Yellow-green solid 178.7mg, yield 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com