Preparation method of (S)-manidipine
A technology of manidipine and resolving agent, applied in the field of preparing S-type manidipine, achieving the effects of low equipment requirements, simple unit operation, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
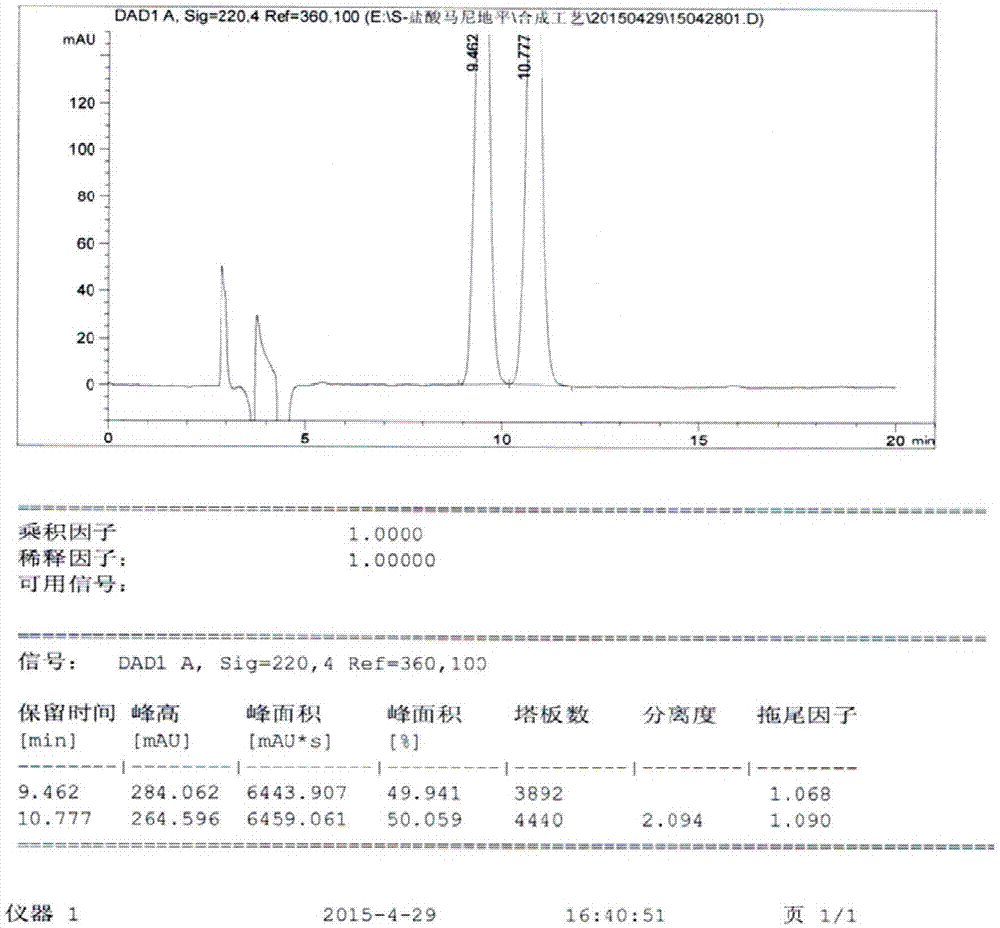
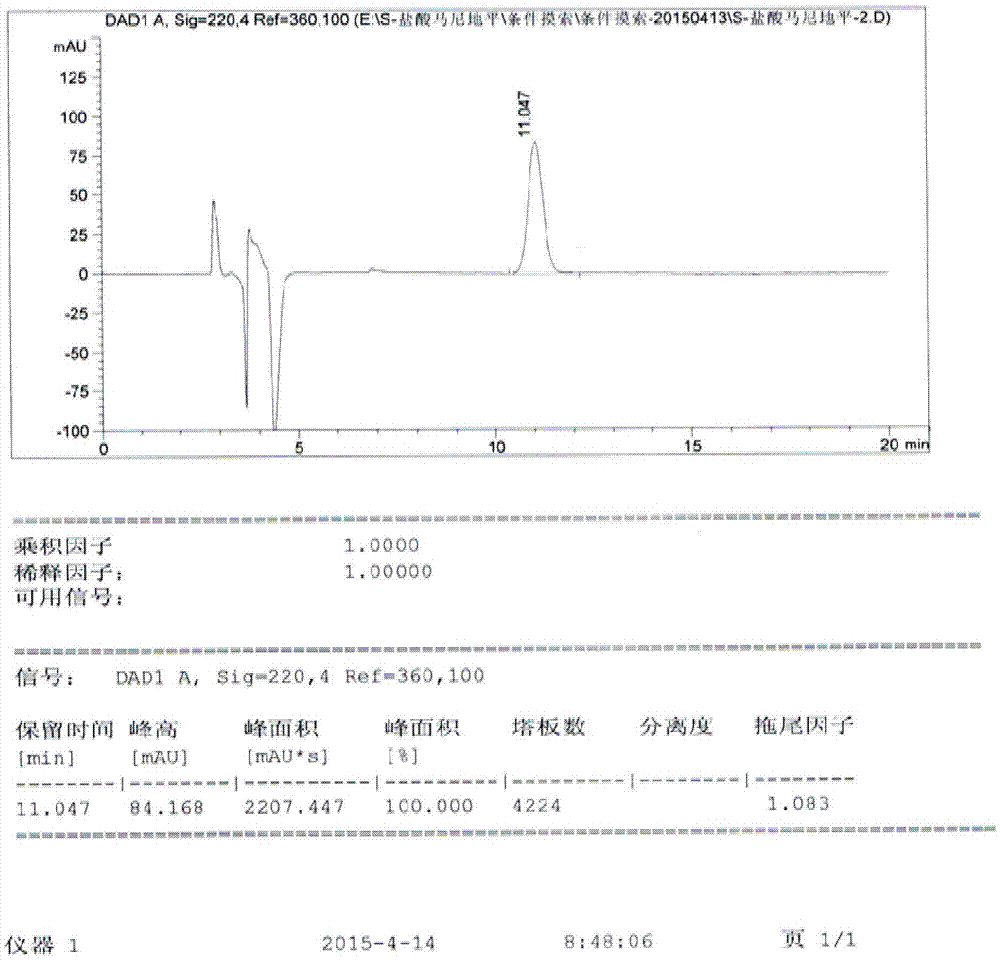
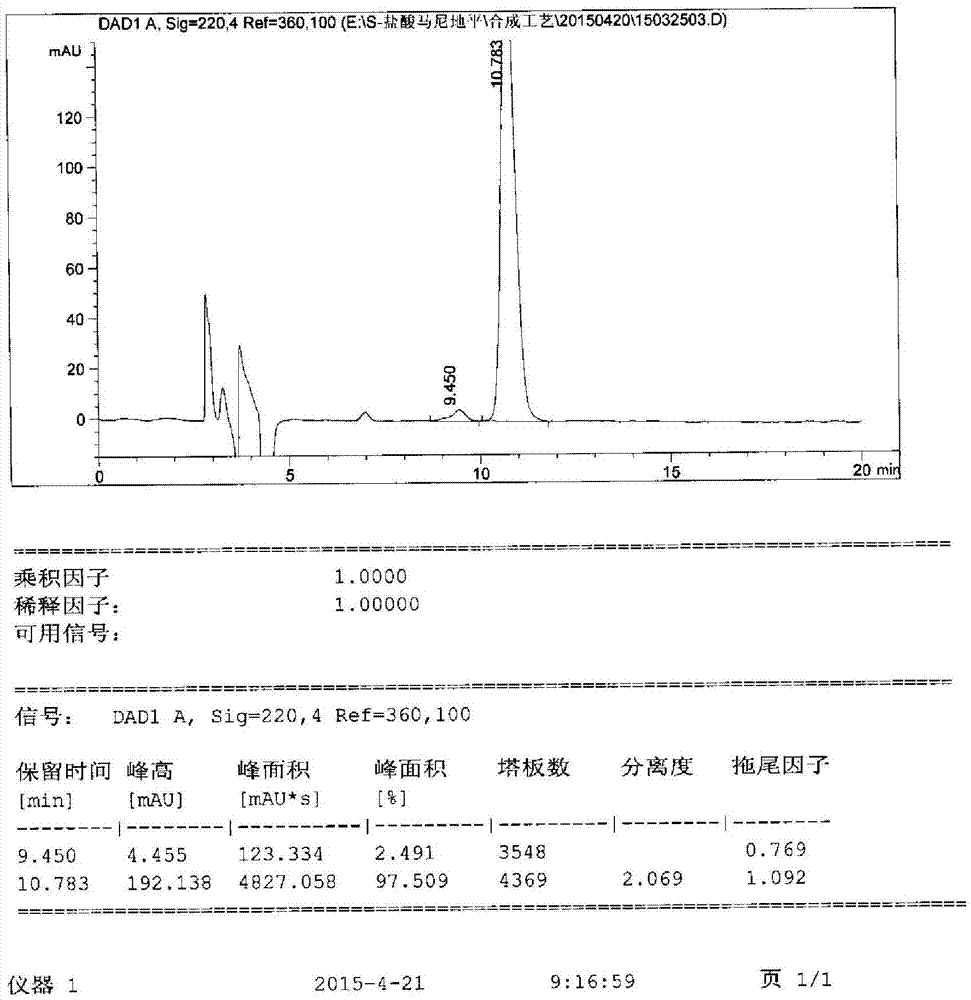
Image
Examples
Embodiment 1
[0040] Embodiment 1: preparation manidipine free base
[0041] Weigh 12 g of sodium hydroxide and add 18 mL of water to prepare a 40% aqueous sodium hydroxide solution, which is cooled in an ice bath.
[0042] Weigh manidipine hydrochloride (20.5 g) into a three-neck flask (500 mL) equipped with mechanical stirring and ice-bath cooling, and then add water (100 mL). Start the stirring, and add the pre-prepared ice-cold 40% sodium hydroxide solution dropwise into the three-neck flask cooled by the ice bath. Ethyl acetate (90 mL) was added during the dropwise addition. After adding all the lye, stop stirring and let stand to separate layers. Take the aqueous phase to measure the pH to 11-12, and separate the liquids. The aqueous phase was extracted twice with ethyl acetate (30 mL×2), and the combined organic phases were washed once with water (50 mL) and saturated sodium chloride solution (50 mL). Then, the organic phase was dried over anhydrous sodium sulfate (15 g) for 2 ho...
Embodiment 2
[0043] Embodiment 2: D-(+)-tartaric acid resolution preparation (S)-manidipine
[0044] Take the manidipine free base (610 mg) prepared in Example 1, add a mixed solvent of acetone and n-heptane (10.0 mL, acetone:n-heptane=2:1) to dissolve. Then, D-(+)-tartaric acid (150 mg) was added into the reaction bottle with stirring, the solution was clear, and then a white solid precipitated out. After continuing to stir for 2 hours, filter and wash the filter cake with a small amount of solvent (acetone:n-heptane=1:1). Then, the obtained filter cake was dried in a vacuum oven at 45° C. for 2 hours to obtain a white solid.
[0045]The obtained white solid was added to 15 mL of ice water, then 15 mL of ethyl acetate was added, and then 40% sodium hydroxide solution was added dropwise to adjust the pH of the aqueous phase to 11-12, and the liquids were separated. The aqueous phase was extracted with ethyl acetate (3×10 mL), the organic phases were combined, washed with 15 mL of water...
Embodiment 3
[0046] Example 3: Preparation of (S)-manidipine by resolution of D-(+)-dibenzoyltartaric acid
[0047] Take the manidipine free base (610 mg) prepared in Example 1, add methanol and n-heptane mixed solvent (10.0 mL, methanol:n-heptane=2:1) to dissolve. Then, D-(+)-dibenzoyltartaric acid (358 mg) was added into the reaction flask with stirring, the solution was clear, and then a white solid precipitated out. After continuing to stir for 2 hours, filter and wash the filter cake with a small amount of solvent (methanol:n-heptane=1:1). Then, the obtained filter cake was dried in a vacuum oven at 45° C. for 2 hours to obtain a white solid.
[0048] The obtained white solid was added to 15 mL of ice water, then 15 mL of ethyl acetate was added, and then 40% sodium hydroxide solution was added dropwise to adjust the pH of the aqueous phase to 11-12, and the liquids were separated. The aqueous phase was extracted with ethyl acetate (3×10 mL), the organic phases were combined, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com