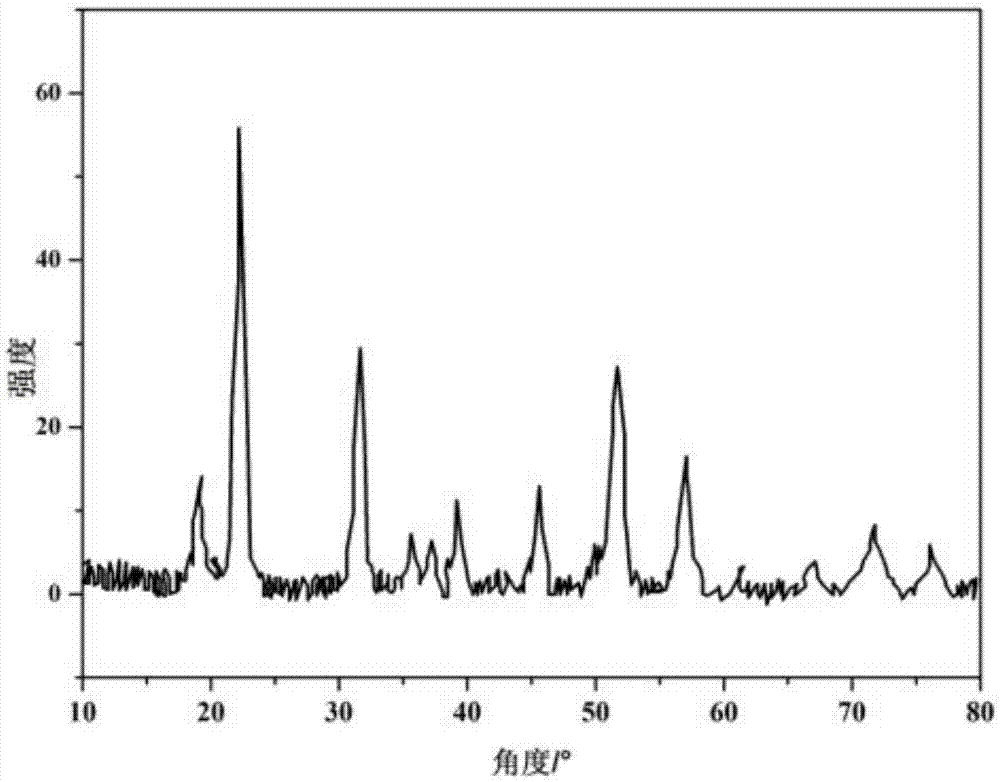
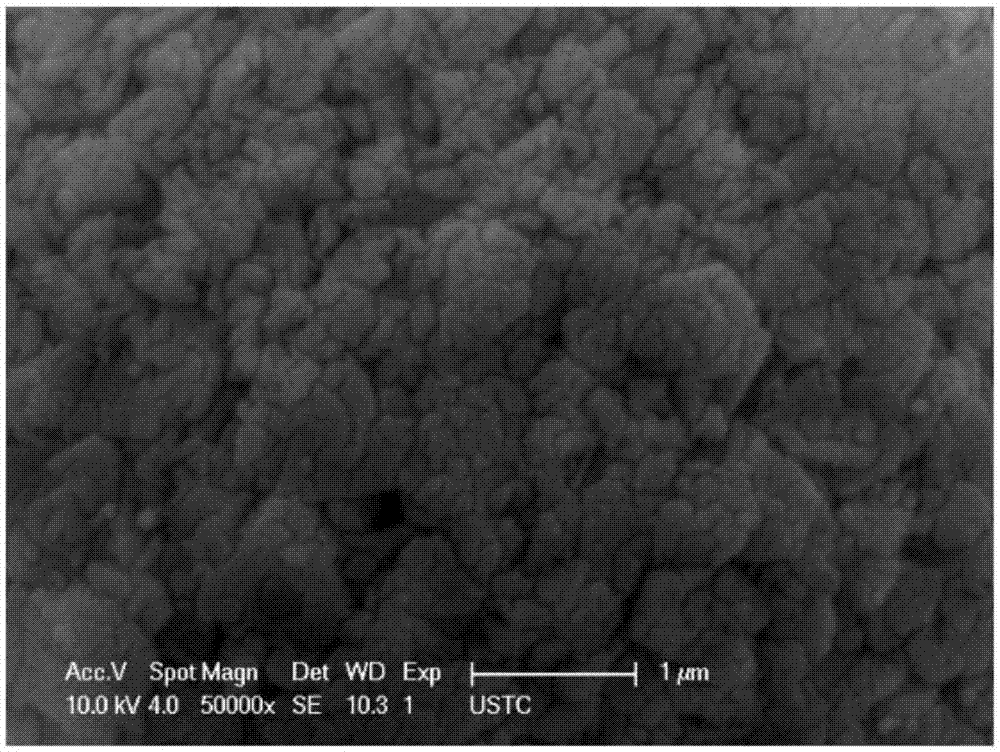
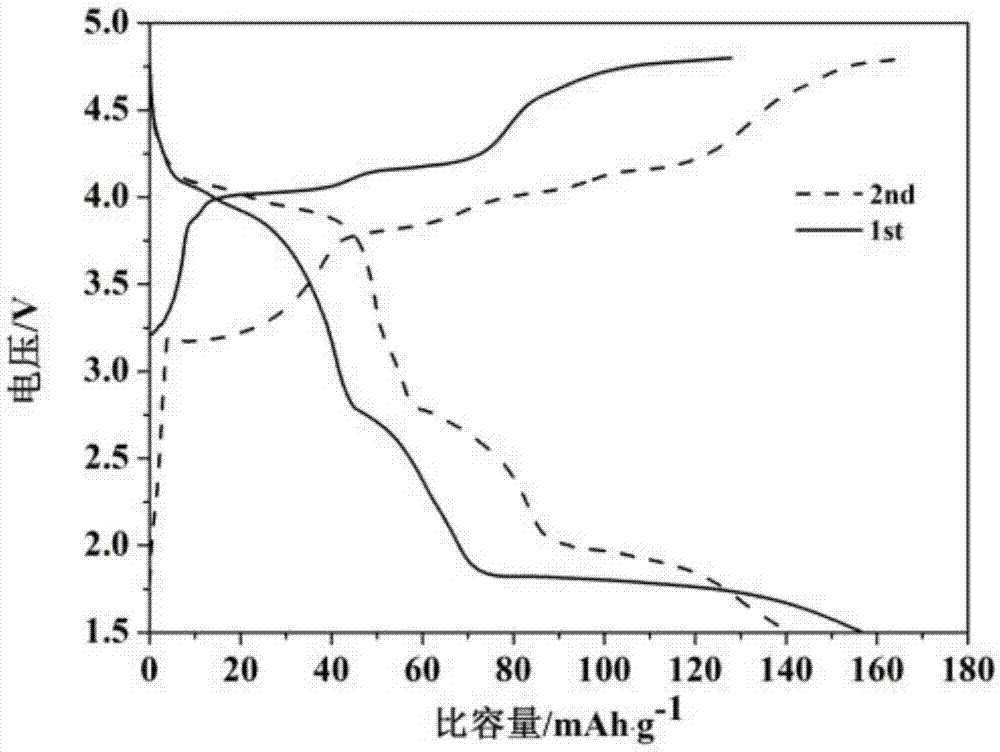
Synthesis method of novel cathode material nano-lithium manganese stannate for lithium-ion battery
A technology for lithium-ion batteries and cathode materials, applied in battery electrodes, nanotechnology for materials and surface science, tin compounds, etc., can solve problems such as inability to mass-produce, achieve high equipment utilization, process control, shorten The effect of the transmission channel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] ⑴Weigh 49.02g of manganese acetate according to the element molar ratio Mn:Sn=1:1.5 and add it to 300mL deionized water to make a transparent solution, weigh 80.02g of sodium stannate and add it to 100mL of deionized water to obtain a concentrated sodium stannate solution. The sodium stannate solution was added dropwise to the manganese acetate solution to form a precipitate, and the precipitate was separated by suction filtration, washed with deionized water and absolute ethanol, and baked in a vacuum oven at 120°C for 12 hours to obtain MnSn(OH) 6 Precursor material;
[0030] ⑵ will get MnSn(OH) 6 Precursor material in N 2 Atmosphere, calcination at 600°C for 24h to obtain nanometer MnSnO 3 Material;
[0031] (3) According to the molar ratio of MnSnO 3 : Li=1:1.2 Weigh 33.24g nanometer MnSnO 3 Materials and 6.65g of lithium carbonate, adding 60g of ethanol as a dispersion medium, 0.4g of PEG20000 as a dispersant, prepared into a uniform slurry with a solid conten...
Embodiment 2
[0035] (1) Weigh 30.20g of manganese sulfate according to the elemental molar ratio Mn:Sn=1:1 and add it to 300mL deionized water to prepare a transparent solution, weigh 53.35g of sodium stannate and add it to 100mL of deionized water to obtain a concentrated sodium stannate solution. The sodium stannate solution was added dropwise to the manganese acetate solution to form a precipitate, and the precipitate was separated by suction filtration and washed with deionized water and absolute ethanol, then baked in a vacuum oven at 100°C for 24 hours to obtain MnSn(OH) 6 Precursor material;
[0036] ⑵ will get MnSn(OH )6 Precursor material in N 2 atmosphere, calcination at 400°C for 12 hours to obtain nanometer MnSnO 3 Material;
[0037] (3) According to the molar ratio of MnSnO 3 : Li=1:1.2 Weigh 33.24g nanometer MnSnO 3Materials and 6.65g of lithium carbonate, adding 27g of ethylene glycol as a dispersion medium, 0.2g of PEG4000 as a dispersant, prepared into a uniform slurr...
Embodiment 3
[0041] (1) Weigh 49.02g of manganese acetate according to the element molar ratio Mn:Sn=1:1 and add it to 300mL deionized water to prepare a transparent solution, weigh 53.35g of sodium stannate and add it to 100mL of deionized water to obtain a concentrated sodium stannate solution. The sodium stannate solution was added dropwise to the manganese acetate solution to form a precipitate, and the precipitate was separated by suction filtration, washed with deionized water and absolute ethanol, and baked in a vacuum oven at 100°C for 12 hours to obtain MnSn(OH) 6 Precursor materials;
[0042] ⑵ will get MnSn(OH) 6 Precursor material in N 2 atmosphere, calcination at 400°C for 24h to obtain nano-MnSnO 3 Material;
[0043] (3) According to the molar ratio of MnSnO 3 : Li=1:1 Weigh 33.24g nanometer MnSnO 3 Materials and 5.54g of lithium carbonate, adding 27g of ethanol as a dispersion medium, 0.4g of PEG20000 as a dispersant, prepared into a uniform slurry with a solid content ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com