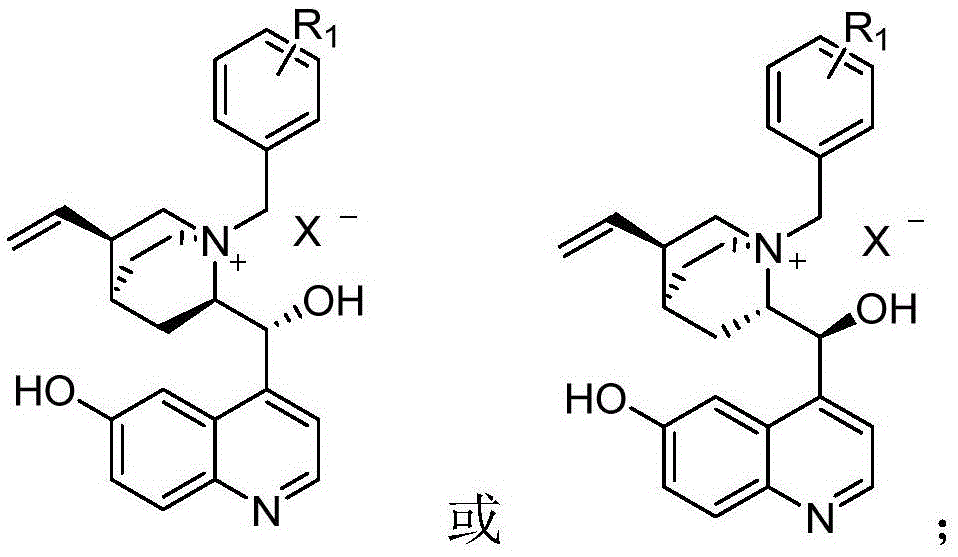
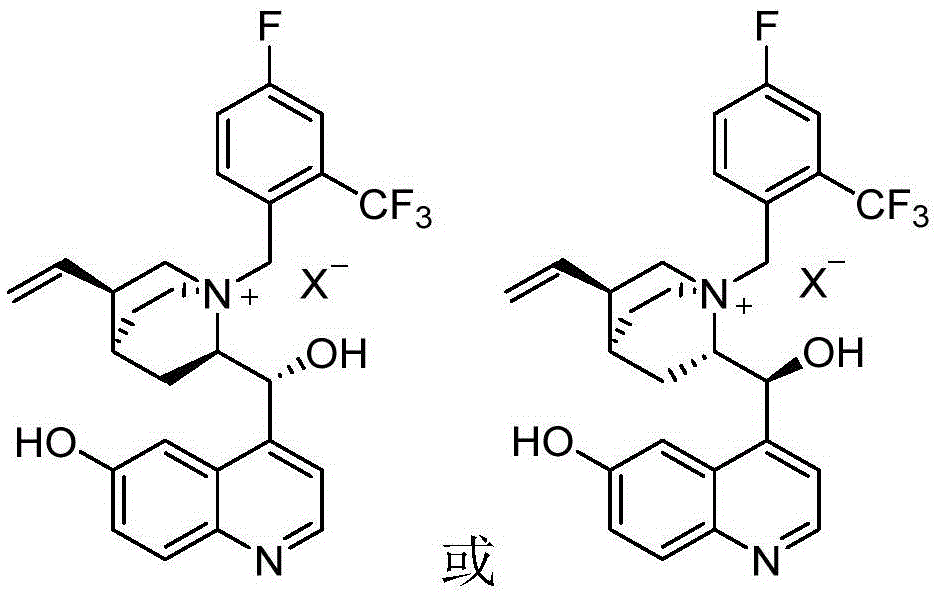
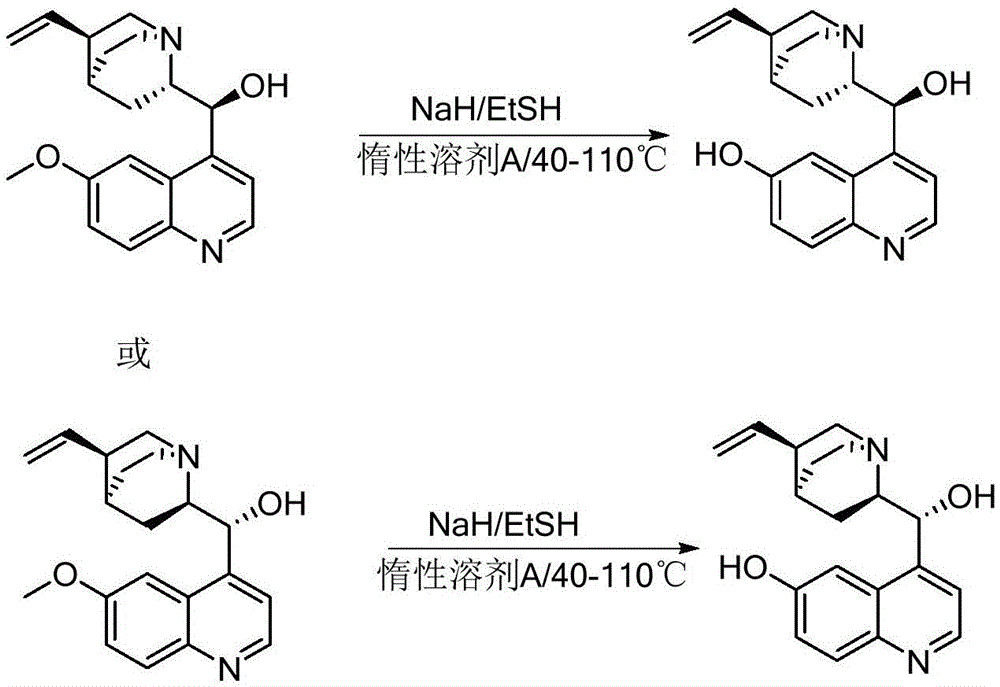
6-hydroxyl quinine quaternary ammonium salt asymmetric phase transfer catalyst, preparation method and application of 6-hydroxyl quinine quaternary ammonium salt asymmetry phase transfer catalyst
A technology of phase transfer catalyst and chinatine, which is applied in the directions of catalytic reaction, chemical instrument and method, preparation of organic compounds, etc., can solve the problems such as application that has not been publicly reported, low enantioselectivity, etc., and achieves mild reaction conditions, The effect of high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of 6-hydroxyl-N-(4-fluoro-2 trifluoromethylbenzyl) quinine quaternary ammonium bromide
[0040]Add 0.5 g (1.6 mmol) of 6-hydroxy-quinine and 0.49 g (1.9 mmol) of p-fluoro-o-trifluoromethylbenzyl bromide into a 50 ml three-necked flask, and add 20 ml of anhydrous THF. The system was heated to 75°C, and the reaction was tracked by TLC. After 6 hours of reflux reaction, the system was cooled, and 30 ml of diethyl ether was added, and the system had obvious solid precipitation. It was filtered, washed three times with ether to obtain a brown solid, and dried to obtain 0.68 g of the product, with a yield of 75%.
[0041] 1 HNMR (500MHz, Chloroform-d) δ8.63 (d, J = 4.5Hz, 1H), 8.45 (dd, J = 10.1, 4.4Hz, 1H), 7.91 (s, 1H), 7.67–7.60 (m, 2H ),7.30(m,1H),7.22(s,1H),6.86(m,1H),6.36(s,1H),5.71(s,1H),5.60–5.51(m,1H),5.48(m, 1H), 5.42(d, J=17.5Hz, 1H), 5.00(dd, J=10.3, 1.8Hz, 1H), 4.57(d, J=9.8Hz, 1H), 4.36(s, 2H), 3.04( dd,J=12.4,10.4Hz,1H),2.86(t,J=1...
Embodiment 2
[0042] Embodiment 2: Preparation of 6-hydroxyl-N-(2-trifluoromethylbenzyl) quinine quaternary ammonium bromide
[0043] 0.5 g (1.6 mmol) of 6-hydroxy-quinine and 0.45 g (1.9 mmol) of m-trifluoromethylbenzyl bromide were added to a 50 ml three-necked flask, and 20 ml of anhydrous dichloromethane was added. The system was heated to 75°C, and the reaction was tracked by TLC. After reflux for 6 hours, cool down, add 20ml of diethyl ether, filter, wash with diethyl ether 3 times to obtain a brown solid, and dry to obtain 0.85g of the product, with a yield of 82%.
[0044] 1 HNMR (500MHz, Chloroform-d) δ8.76(s, 1H), 8.66(d, J=4.6Hz, 1H), 7.99(s, 1H), 7.74(d, J=4.9Hz, 2H), 7.68( s,1H),7.53(d,J=9.1Hz,1H),7.39(d,J=7.8Hz,1H),7.24(s,1H),6.71(d,J=9.1Hz,1H),6.29( s,1H),6.09(d,J=12.1Hz,1H),5.67(s,1H),5.51–5.44(m,1H),5.41(dd,J=10.0,5.1Hz,1H),5.36(d ,J=17.4Hz,1H),5.00(m,1H),4.60(d,J=10.0Hz,1H),4.20(s,1H),3.96(d,J=12.8Hz,1H),3.01(t ,J=11.6Hz,1H),2.89(t,J=11.8Hz,1H),2.51(s,1H),2.09(t,J=12....
Embodiment 3
[0045] Example 3: Preparation of 6-hydroxy-N-(1,2,3,4,5-pentafluoro-benzyl) quinine quaternary ammonium bromide
[0046] Add 0.5 g (1.6 mmol) of 6-hydroxy-quinine and 0.52 g (1.9 mmol) of 1,2,3,4,5-pentafluoro-benzyl bromide into a 50 ml three-necked flask, and add 20 ml of anhydrous THF. The system was heated to 75°C, and the reaction was tracked by TLC. After reflux for 6 hours, cool down, add 20 ml of diethyl ether, filter, wash with diethyl ether three times to obtain a brown solid, and dry to obtain 0.62 g of the product, with a yield of 76%.
[0047] 1 HNMR (500MHz, DMSO-d 6 )δ10.15(s,1H),8.73(d,J=4.5Hz,1H),7.95(d,J=9.1Hz,1H),7.67(d,J=4.4Hz,1H),7.57–7.33( m,2H),6.75–6.54(m,1H),6.37(t,J=2.6Hz,1H),5.77(s,1H),5.42(d,J=13.5Hz,1H),5.14(dt,J =17.2,1.3Hz,1H),5.03(dt,J=10.4,1.2Hz,1H),4.91(d,J=13.4Hz,1H),4.17(d,J=7.5Hz,2H),4.08(q ,J=5.2Hz,2H),3.66–3.58(m,4H),2.68–2.57(m,1H),2.18(t,J=10.5Hz,2H),2.01(q,J=3.2Hz,1H) ,1.76(dq,J=6.5,3.1Hz,4H),1.53–1.27(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com