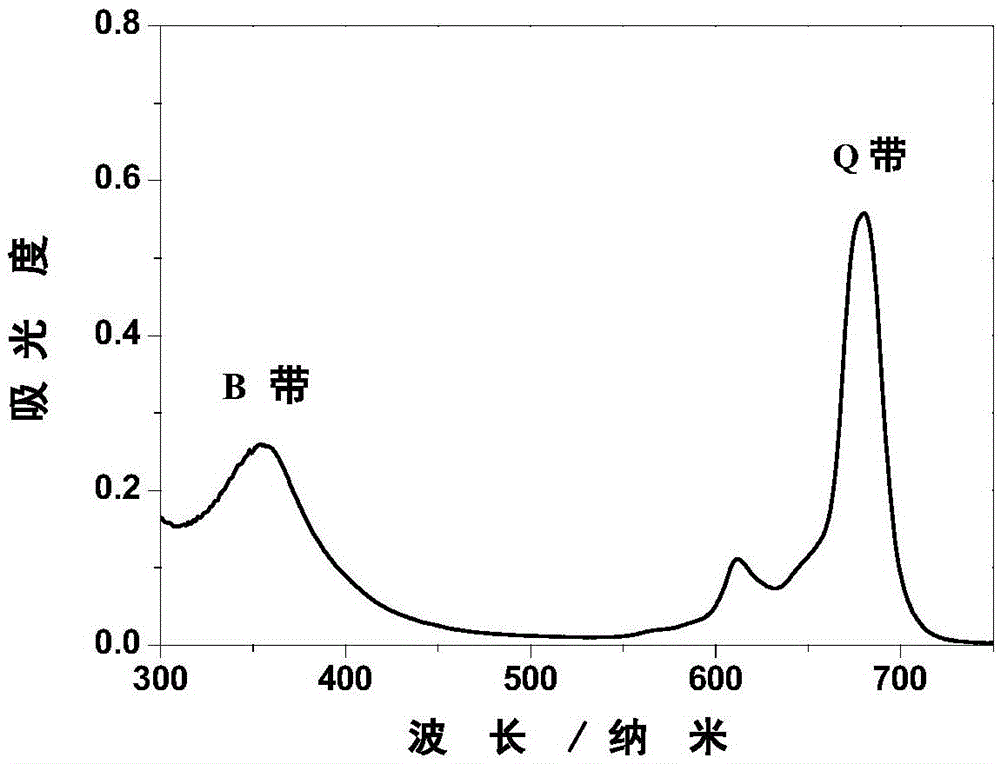
Amphiphilic zinc phthalocyanine and preparation method and application thereof
A zinc phthalocyanine, amphiphilic technology, applied in the field of photosensitizers, can solve the problem of unsatisfactory site specificity and achieve good singlet oxygen generation rate, high anti-tumor activity, and reduce the degree of aggregation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of an amphiphilic zinc phthalocyanine photosensitizer, the steps are as follows:
[0042] (1) Under nitrogen protection, 4-nitrophthalonitrile, p-hydroxybenzylamine and K 2 CO 3 Add it into the reaction flask at a molar ratio of 1:1.5:2, use 10ml of anhydrous DMF as a solvent, and react at 40-50°C for 5h to obtain phthalonitrile intermediate 1.
[0043] (2) Under the protection of nitrogen, the phthalonitrile intermediate 1 was stirred and dissolved in absolute ethanol, and mixed with 1,3-propane sultone at a molar ratio of 1:1.3 at room temperature for 1 hour, and then heated to 80 After reacting at ℃ for 6 hours, 15 ml of acetone was added to continue stirring for 3 hours, and the phthalonitrile intermediate 2 was obtained by filtration.
[0044] (3) Under the protection of nitrogen, phthalonitrile intermediate 2, zinc acetate and DBU were added to n-pentanol in a molar ratio of 4:2.5:2.5, refluxed for 24 hours, and poured into acetone after coo...
Embodiment 2
[0047] A preparation method of an amphiphilic zinc phthalocyanine photosensitizer, the steps are as follows:
[0048] (1) Under nitrogen protection, 4-nitrophthalonitrile, p-hydroxybenzylamine and K 2 CO 3 Add it into the reaction flask at a molar ratio of 1:1:1.5, use 10ml of anhydrous DMF as a solvent, and react at 50-60°C for 5h to obtain phthalonitrile intermediate 1.
[0049] (2) Under the protection of nitrogen, the phthalonitrile intermediate 1 was stirred and dissolved in absolute ethanol, and mixed with 1,3-propane sultone at a molar ratio of 1:1.1 at room temperature for 1 hour, and then heated to 80 After reacting at ℃ for 6 hours, 15 ml of acetone was added to continue stirring for 3 hours, and the phthalonitrile intermediate 2 was obtained by filtration.
[0050] (3) Under the protection of nitrogen, phthalonitrile intermediate 2, zinc acetate and DBU were added to n-pentanol in a molar ratio of 4:2.5:2.5, refluxed for 24 hours, and poured into acetone after cooli...
Embodiment 3
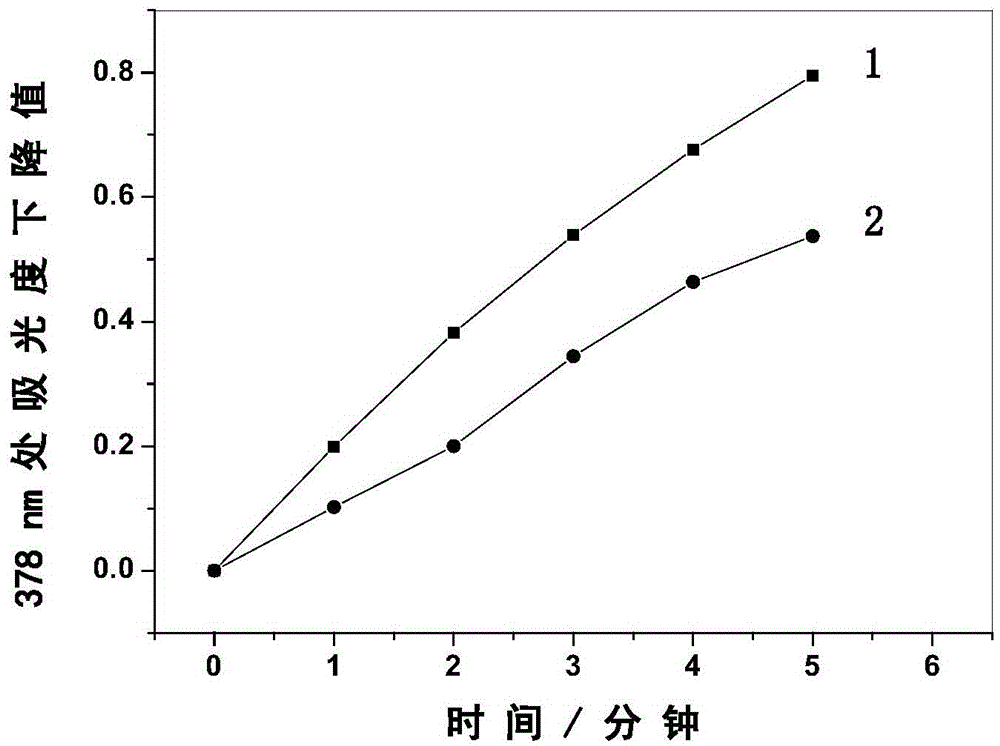
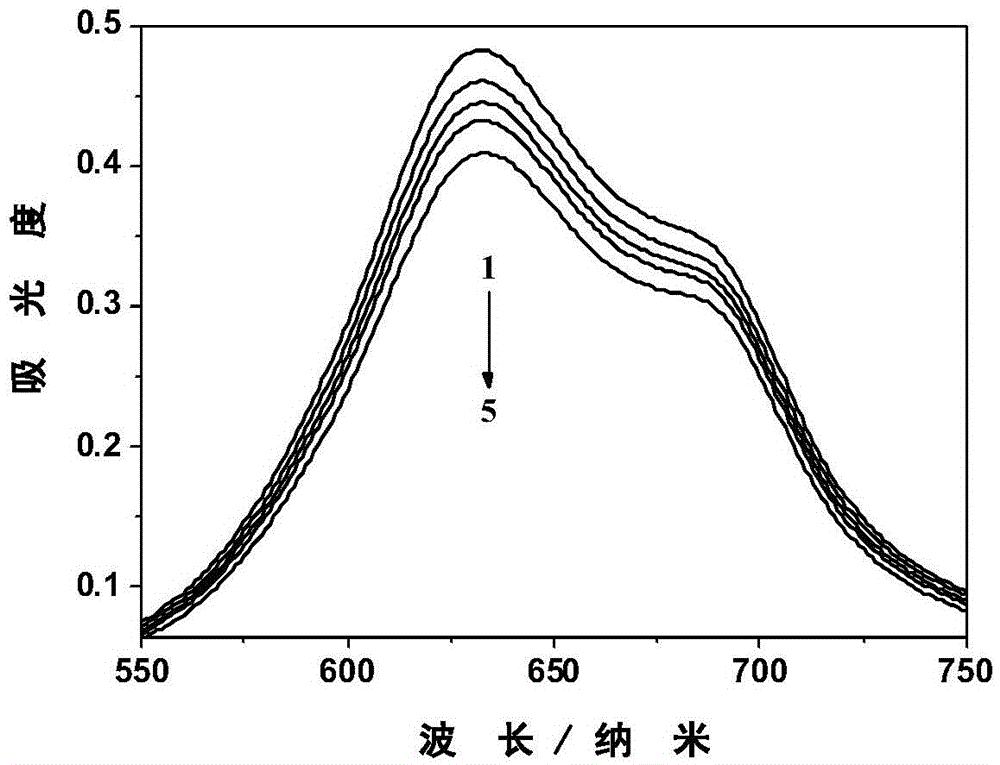
[0052] The amphiphilic zinc phthalocyanine and hematoporphyrin aqueous solution containing ADPA were irradiated with 665nm LED lamp respectively, and by detecting its ultraviolet-visible absorption spectrum, it can be found that with the prolongation of the illumination time, the characteristic of ADPA in the two solutions is located at 378nm. The peak intensity of the absorption peak is continuously decreasing, and the relationship curve of the decline value of the characteristic absorption peak of ADPA at 378nm is obtained as the light time changes. By comparison, it is found that the singlet oxygen production rate of the amphiphilic zinc phthalocyanine is higher than that of hematoporin phylloline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com