5-fluoro polysubstituted pyrroline derivative and preparation method thereof
A technology of dihydropyrrole and multi-substitution, applied in the direction of organic chemistry, etc., can solve the problem of less literature on the synthesis of fluorine-containing dihydropyrrole, and achieve the effects of mild reaction conditions, simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
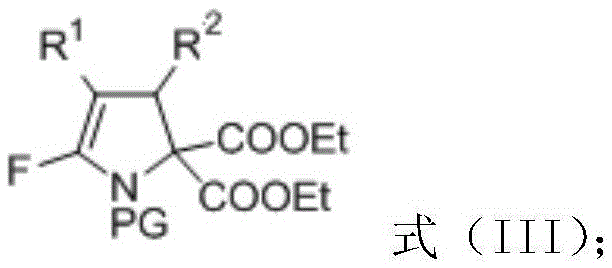
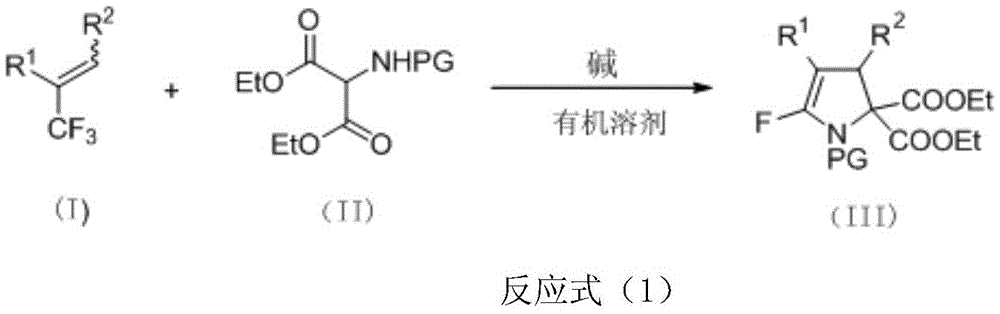
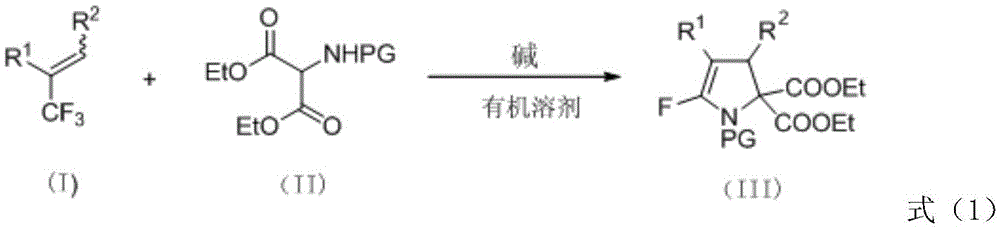
[0038] Put raw materials 3-(4-bromophenyl)-2-(trifluoromethyl)ethyl acrylate (0.45mmol), diethyl p-toluenesulfonamidomalonate (0.3mmol) into a pre-activated Molecular sieves and formula (II) in the drying reaction tube of potassium carbonate of 1.2 times of the amount of amidodiethyl malonate substance, fully react at room temperature, react for 6 hours, detect reaction by TLC and iodine cylinder color development, to the right Diethyl tosylaminomalonate disappeared completely. Add 5.0ml of distilled water after the reaction to quench the reaction, extract with diethyl ether or ethyl acetate (3*5.0ml), combine the organic phases to dry and remove the solvent by rotary evaporation, and then directly purify the crude product by silica gel flash column chromatography (petroleum ether : ethyl acetate=4:1) to obtain the pure product III-1 of 5-fluoro polysubstituted dihydropyrrole derivative (156 mg, 82%).
[0039]
[0040] Yellow oily substance. 1 HNMR (400MHz, CDCl 3 )δ8.06...
Embodiment 2
[0042] With 3-(4-bromophenyl)-2-(trifluoromethyl)ethyl acrylate (0.45mmol), diethyl acetamidomalonate (0.3mmol) as raw material, other operation reference example 1, reaction Stir for 6h, and purify by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the pure 5-fluoro polysubstituted dihydropyrrole derivative III-2 (18 mg, 12%).
[0043]
[0044] Colorless oil. 1 HNMR (500MHz, CDCl 3 )δ7.41(d,J=7.6Hz,2H),7.12(brs,2H),4.60(d,J=2.9Hz,1H),4.39-4.33(m,2H),4.21–3.96(m,1H ),3.74–3.44(m,1H),2.40(d,J=4.9Hz,3H),1.37(t,J=5.9Hz,3H),1.12(t,J=5.9Hz,3H),0.89(t ,J=6.0Hz,3H). 19 FNMR (282MHz, CDCl 3 )δ-99.39. 13 CNMR (126MHz, CDCl 3 )δ166.72(d, J=3.7Hz), 166.45, 162.74, 162.05(d, J=5.8Hz), 155.34(d, J=295.4Hz), 135.40, 135.39, 131.17, 122.24, 88.03(d, J =5.0Hz), 73.80, 63.12, 62.17, 60.51, 48.93, 23.71 (d, J = 11.2Hz), 14.02, 13.94, 13.33. HRMS (ESI) calcdforC 21 h 23 BrFNNaO 7 [M+Na + ]:522.0534,found:522.0541.
Embodiment 3
[0046] Use ethyl 3-(4-bromophenyl)-2-(trifluoromethyl)acrylate (0.45mmol), diethyl p-nitrobenzenesulfonamidomalonate (0.3mmol) as raw materials, and other operations refer to Example 1, the reaction was stirred for 4 hours, purified by silica gel column chromatography (petroleum ether: ethyl acetate = 4:1), and the pure 5-fluoro polysubstituted dihydropyrrole derivative III-3 (79mg, 41%) was obtained.
[0047]
[0048] Yellow oily substance. 1 HNMR (300MHz, CDCl 3 )δ8.41(s,4H),7.43(d,J=8.6Hz,2H),7.11(brs,2H),4.83(d,J=5.9Hz,1H),4.65–4.36(m,2H), 4.28–3.93(m,2H),3.91–3.60(m,2H),1.46(t,J=7.1Hz,3H),1.11(t,J=7.1Hz,3H),1.05(t,J=7.2Hz ,3H). 19 FNMR (282MHz, CDCl 3 )δ-97.31. 13 CNMR (101MHz, CDCl 3 )δ166.85,163.36,161.41(d,J=5.7Hz),155.09(d,J=295.0Hz),150.75,144.51,134.44,131.29,130.62,130.60,124.08,122.64,87.07(d,J=3.8Hz) ,77.73,63.8,63.15,60.69,50.35,13.99,13.96,13.35.HRMS(ESI)calcdforC 25 h 24 BrFN 2 NaO 10 S[M+Na + ]:665.0211,found:665.0219.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com