Method for synchronously extracting and separating uranium and molybdenum
An extraction and volume percentage technology, applied in the field of hydrometallurgy, can solve the problems of high investment, increase the difficulty and cost of wastewater treatment, and achieve the effects of reducing accumulation, reducing the amount of suspended solids and reducing consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
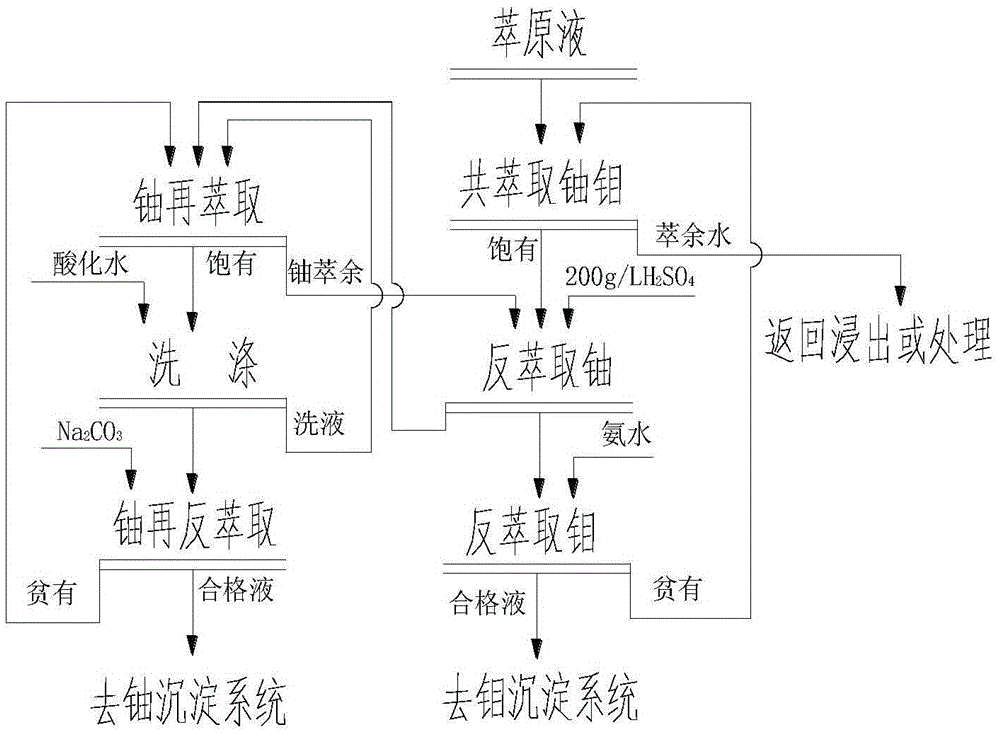
[0020] Extraction solution: pH0.73, ρ(U)0.64 / L, ρ(Mo)8.12g / L, ρ(SiO 2 )0.25g / L, ρ(SO 4 2- ) 121.9g / L, ρ(Al) 1.13g / L, ρ(∑Fe) 2.24g / L, Eh-410mV.
[0021] Co-extracted uranium and molybdenum organic phase: 7.5% N235+20% TBP+72.5% sulfonated kerosene
[0022] Co-extraction of uranium and molybdenum operating conditions: comparison: O / A=3, flow ratio A / O=1.5, contact time 3min.
[0023] Organic phase loaded with uranium and molybdenum: ρ(Mo) 12.1g / L, ρ(U) 0.94g / L
[0024] Uranium molybdenum raffinate aqueous phase: ρ(Mo)<20mg / L, ρ(U)<5mg / L
[0025] Molybdenum-uranium-loaded organic reverse extraction of uranium: stripping agent: 200g / L sulfuric acid solution
[0026] Operating conditions for stripping uranium: comparison: O / A=1.5, flow ratio A / O=4, contact time 3min
[0027] Uranium stripping solution: ρ(U)~3.7g / L
[0028] Re-extracted uranium organic phase composition: 5% P204+5% TBP+90% sulfonated kerosene
[0029] Operating conditions for re-extracting uranium: compariso...
Embodiment 2
[0037] Extraction solution: pH0.64, ρ(U)0.667 / L, ρ(Mo)6.28g / L, ρ(SiO 2 )0.29g / L, ρ(SO 4 2- ) 101.9g / L, ρ(Al) 1.28g / L, ρ(∑Fe) 1.934g / L, Eh-408mV.
[0038] Co-extracted uranium and molybdenum organic phase: 7.5% N235+20% TBP+72.5% sulfonated kerosene
[0039] Co-extraction of uranium and molybdenum operating conditions: comparison: O / A=3, flow ratio A / O=2, contact time 3min.
[0040] Organic phase loaded with uranium and molybdenum: ρ(Mo)~12.5g / L, ρ(U)~1.31g / L
[0041] Uranium molybdenum raffinate aqueous phase: ρ(Mo)<20mg / L, ρ(U)<5mg / L
[0042] Molybdenum-uranium-loaded organic reverse extraction of uranium: stripping agent: 200g / L sulfuric acid solution
[0043] Operating conditions for stripping uranium: comparison: O / A=1.5, flow ratio A / O=3, contact time 3min
[0044] Uranium stripping solution: ρ(U)~3.9g / L
[0045] Re-extracted uranium organic phase composition: 5% P204+5% TBP+90% sulfonated kerosene
[0046] Operating conditions for re-extracting uranium: compariso...
Embodiment 3
[0054]Extraction solution: pH0.84, ρ(U)1.34 / L, ρ(Mo)5.84g / L, ρ(SiO 2 )0.232g / L, ρ(SO 4 2- ) 114.2g / L, ρ(Al) 0.98g / L, ρ(∑Fe) 2.38g / L, Eh-389mV.
[0055] Co-extracted uranium and molybdenum organic phase: 7.5% N235+20% TBP+72.5% sulfonated kerosene
[0056] Co-extraction of uranium and molybdenum operating conditions: comparison: O / A=3, flow ratio A / O=2, contact time 3min.
[0057] Organic phase loaded with uranium and molybdenum: ρ(Mo)~11.6g / L, ρ(U)~2.6g / L
[0058] Uranium molybdenum raffinate aqueous phase: ρ(Mo)<20mg / L, ρ(U)<5mg / L
[0059] Molybdenum-uranium-loaded organic reverse extraction of uranium: stripping agent: 200g / L sulfuric acid solution
[0060] Operating conditions for stripping uranium: comparison: O / A=1.5, flow ratio A / O=1.5, contact time 3min
[0061] Uranium stripping solution: ρ(U)~3.9g / L
[0062] Re-extracted uranium organic phase composition: 5% P204+5% TBP+90% sulfonated kerosene
[0063] Operating conditions for re-extracting uranium: comparison...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com