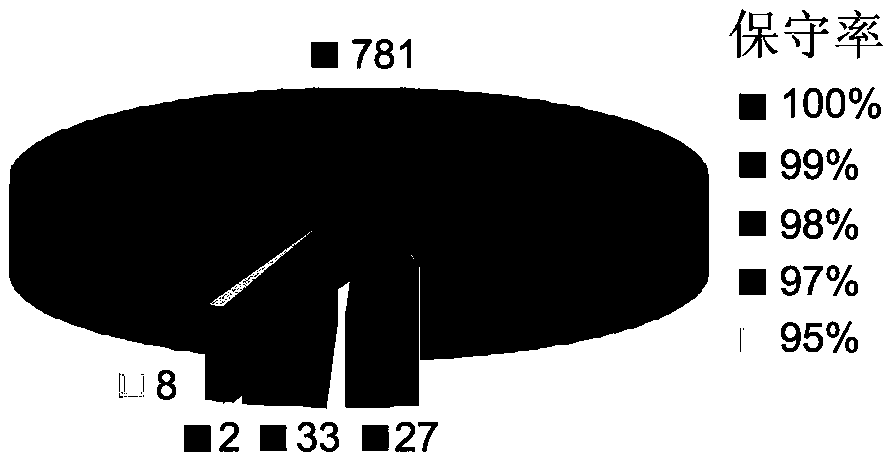
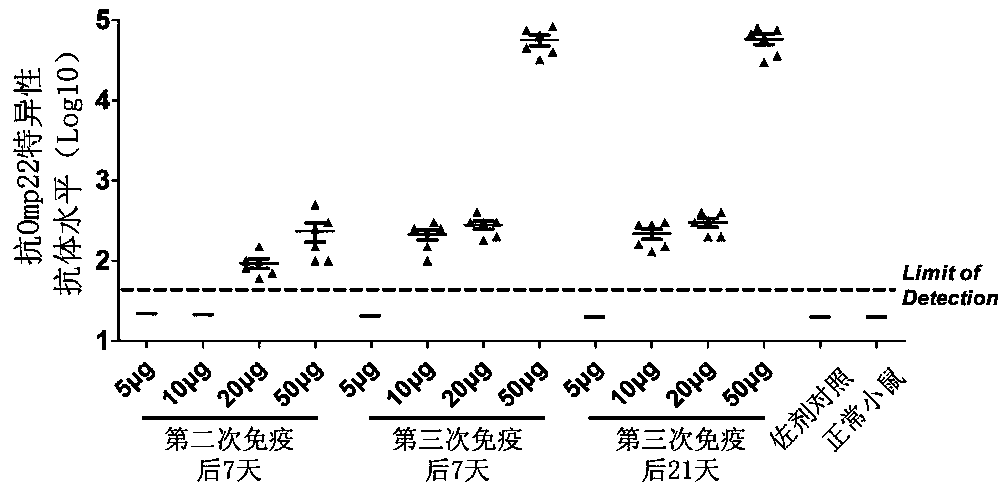
Application of outer membrane protein Omp22 as acinetobacter baumannii vaccine target
A technology of Acinetobacter baumannii and outer membrane protein, which is applied in the fields of molecular biology and immunology, and can solve problems such as difficulty in improving yield, complex mechanism of Baumann's drug resistance, and severe drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Construction of a prokaryotic expression plasmid for the outer membrane protein Omp22 of Acinetobacter baumannii
[0033] The Acinetobacter baumannii strain ATCC17978 was cultured overnight in LB medium until the bacteria grew to the logarithmic phase (OD 600=0.4-0.6), the bacterial liquid was taken for PCR amplification, and the PCR amplification primers were Omp22-F (5'GGATCCATGCGTGCATTAGTTATTT3'); Omp22-R (5'GAATTCTTATTGTTTAGCATAAATG3'); the specific PCR system was: bacterial liquid: 2 μL; Omp22 -F: 2 μL; Omp22-R: 2 μL; dNTP: 2 μL; Taq enzyme: 0.25 μL; 10×Taq enzyme buffer: 2.5 μL; water: 14.25 μL. The PCR program was: ①pre-denaturation at 94°C for 5 minutes; ②denaturation at 94°C for 30 seconds; annealing at 56°C for 30 seconds; extension at 72°C for 1 minute, 35 cycles; ③final extension at 72°C for 10 minutes. The PCR product was gel-recovered (Beijing Tiangen Biology), and the recovered product was connected to the pMD19T-simple vector (Takara Company)...
Embodiment 2
[0034] Example 2: Induced Expression of Acinetobacter baumannii Omp22 Recombinant Protein
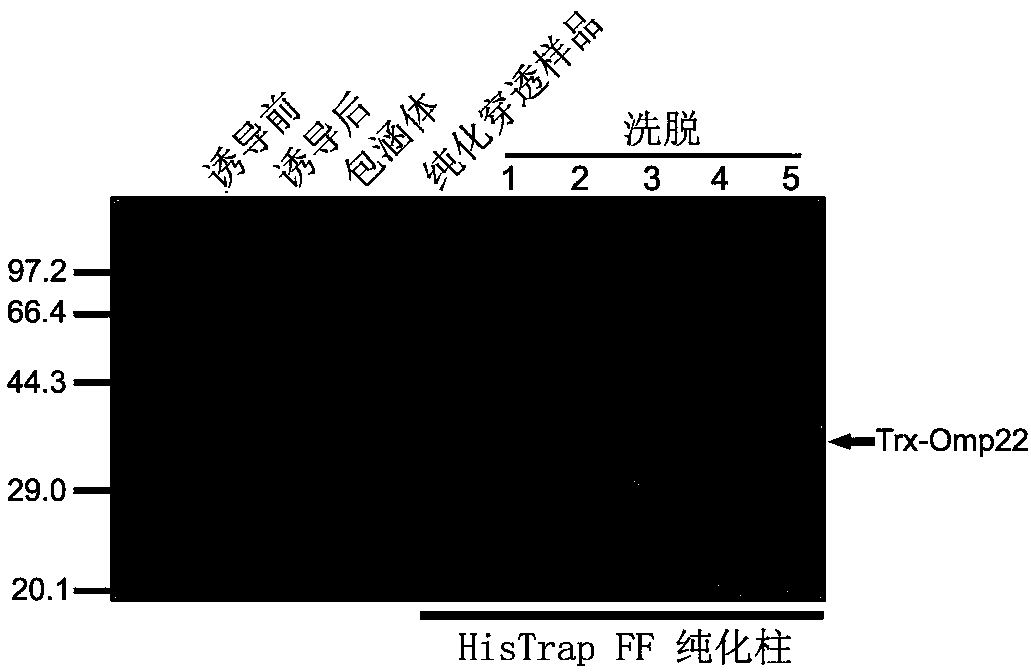
[0035] Transform the recombinant plasmid into BL21(DE3) competent cells, and pick a single clone to induce expression. The specific method is to inoculate the positive clone into LB medium that has been added with ampicillin (100 mg / mL), and culture it for 16 hours. Transfer the bacteria liquid into the new LB medium with ampicillin at a ratio of 1:5. When the strain grows to the logarithmic phase, add IPTG (1mmol / L) to induce expression, control the induction expression temperature at 37°C, and induce Express 6 hours ( figure 2 ).
Embodiment 3
[0036] Example 3: Preparation and purification of Omp22 subunit vaccine antigen
[0037] Centrifuge the sample after overnight induction at 12,000 g at room temperature for 10 minutes to collect the bacteria, wash the bacteria twice with PBS, and then ultrasonically disrupt the cells at 10% maximum power, work for 5 seconds, stop for 5 seconds, and last for 10 minutes. The crushed sample was centrifuged at 12000g for 10 minutes, and the inclusion body precipitate was collected. The inclusion body was washed 3 times with Washbuffer (20mMtris-Hcl, pH8.8, 1M urea) and then added to Solutionbuffer (20mMtris-Hcl, pH8.8, 8M urea). Dissolved, the dissolved inclusion bodies were dialyzed with Bindingbuffer (20mMtris-Hcl, pH8.8) at 4°C overnight for refolding, the dialysate was purified with HisTrapFF (GEHealthcare), and filtered through Elutionbuffer (20mMtris-Hcl, pH8.8, 1M imidazole ) after gradient elution, the purified products of different components were collected and detected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com